Opioid use disorder risk alleles in self-reported assigned African American/Afro-Caribbean and European biogeographical genetic ancestry groups and in males and females
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
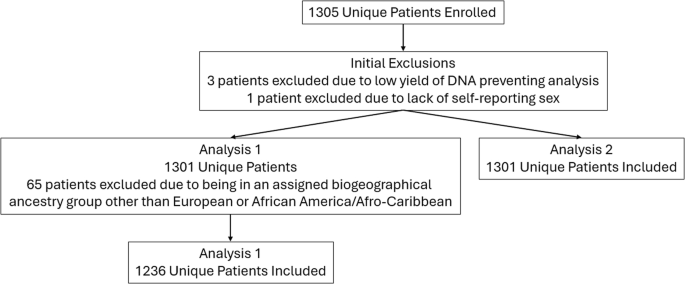
The influence of genetic variants related to opioid use disorder (OUD) was evaluated using multiple logistic regression analysis in self-reported assigned African American/Afro-Caribbean and European biogeographical ancestry groups (BGAGs) and by sex. From a sample size of 1301 adult patients (>18 years of age) seen in emergency departments of three medical centers in Ohio, six variants were found to be associated with OUD. Two of the variants, rs2740574 (CYP3A4) and rs324029 (DRD3), were included in the analysis having met criteria of at least five subjects for each BGAG, variant carrier status, and OUD status combinations. Variant carriers in the African/Afro-Caribbean BGAG had slightly lower predicted probabilities of OUD. Variant carriers in the European BGAG had slightly higher predicted probabilities of OUD. Relative to sex, all the six variants met evaluation criteria (five subjects for all sex, variant, and OUD status combinations). No statistically significant interactions were found between a given variant, BGAGs and sex. Findings suggest variant testing relative to OUD risk can be applied across BGAGs and sex, however, studies in larger populations are needed.
非裔美国人/非裔加勒比人和欧洲人生物地理遗传血统群体以及男性和女性中自我报告的阿片类药物使用障碍风险等位基因。
我们使用多元逻辑回归分析法评估了与阿片类药物使用障碍(OUD)有关的遗传变异对自我报告的非裔美国人/加勒比海非裔美国人和欧洲人生物地理祖先群体(BGAGs)以及性别的影响。在俄亥俄州三家医疗中心急诊科就诊的 1301 名成年患者(18 岁以上)样本中,发现六个变异与 OUD 相关。其中rs2740574 (CYP3A4)和rs324029 (DRD3)这两个变异体在每个BGAG、变异体携带者状态和OUD状态组合中至少有五个受试者符合标准,因此被纳入分析。非洲/非洲-加勒比海 BGAG 变异携带者的 OUD 预测概率略低。欧洲 BGAG 变异携带者的 OUD 预测概率略高。就性别而言,所有六个变异体都符合评估标准(所有性别、变异体和 OUD 状态组合中有五个受试者)。在特定变体、BGAGs 和性别之间没有发现具有统计学意义的交互作用。研究结果表明,与 OUD 风险相关的变异测试可适用于不同的 BGAG 和性别,但还需要在更大的人群中进行研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


