Machine learning thermodynamic perturbation theory offers accurate activation free energies at the RPA level for alkene isomerization in zeolites†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The determination of accurate free energy barriers for reactions catalyzed by proton-exchanged zeolites by quantum chemistry approaches is a challenge. While ab initio molecular dynamics is often required to sample correctly the various states described by the system, the level of theory also has a crucial impact. In the present work, we report the determination of accurate barriers for a type B isomerization of a monobranched C7 alkene (4-methyl-hex-1-ene) into a dibranched tertiary cation inside a protonated chabazite zeolite. This is done by using the Machine Learning Thermodynamic Perturbation Theory (MLPT) at the Random Phase Approximation (RPA) level, on the basis of blue-moon sampling dynamic data obtained at the Generalized Gradient Approximation (GGA) level (PBE+D2). The comparison of PBE+D2 and RPA profiles shows that the former overstabilizes cationic intermediates with respect to neutral ones. The transition state of the isomerization is a non-classical edge protonated cyclopropane, the stabilization of which is lower than that of the π-complex when PBE+D2 is replaced by RPA, but higher than that of the classical tertiary carbenium. Consequently, the backward isomerization barrier is decreased. Applying the MLPT approach to recompute the free energy barriers with various dispersion correction schemes to the PBE energies shows that none of the schemes is sufficient to improve both the forward and backward barriers with respect to the RPA reference. These data complement previously determined alkene cracking barriers [Rey et al., Angew. Chem., Int. Ed., 2024, 63, e202312392], thanks to which it is possible to compare the presently determined barriers with reference experimental data [Schweitzer et al., ACS Catal., 2022, 12, 1068–1081]. The agreement with experiments is significantly improved at the RPA with respect to GGA. Chemical accuracy is approached (maximum deviation of 6.4 kJ mol−1), opening the door to predictive kinetic modelling starting from first principles approaches.

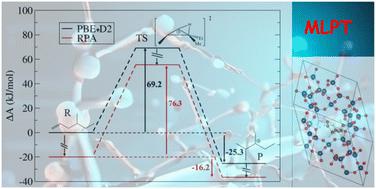
机器学习热力学扰动理论在 RPA 水平上为沸石中的烯异构化提供了精确的活化自由能
用量子化学方法准确测定质子交换沸石催化反应的自由能垒是一项挑战。要正确采样系统所描述的各种状态,通常需要进行 ab initio 分子动力学,但理论水平也会产生重要影响。在本研究中,我们报告了在质子化夏巴沸石内将单支链 C7 烯(4-甲基-1-己烯)异构化为二支链叔阳离子的 B 型异构化精确壁垒的测定结果。这是通过随机相近似(RPA)水平的机器学习热力学扰动理论(MLPT),在广义梯度近似(GGA)水平(PBE+D2)获得的蓝月采样动态数据的基础上实现的。PBE+D2 与 RPA 曲线的比较表明,前者使阳离子中间体比中性中间体更加稳定。异构化的过渡态是非经典的边缘质子化环丙烷,当 PBE+D2 被 RPA 取代时,其稳定度低于 π 复合物的稳定度,但高于经典的叔碳鎓的稳定度。因此,反向异构化障碍降低了。用 MLPT 方法重新计算自由能垒,并对 PBE 能量采用各种分散校正方案,结果表明,与 RPA 参考值相比,没有一种方案足以改善前向和后向自由能垒。这些数据补充了之前确定的烯裂解势垒[Rey 等人,Angew. Chem.,Int. Ed.,2024,63,e202312392],由于这些数据,可以将目前确定的势垒与参考实验数据进行比较[Schweitzer 等人,ACS Catal.,2022,12,1068-1081]。与 GGA 相比,RPA 与实验的一致性明显提高。化学精确度已经接近(最大偏差为 6.4 kJ mol-1),为从第一原理方法开始的预测动力学建模打开了大门。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


