Rearrangment–Cyclization of Dialkyl(4-hydroxybut-2-ynyl)(3-phenylprop-2-enyl)ammonium Bromides in the Presence of Aqueous Alkali
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Dialkyl(4-hydroxybut-2-ynyl)(3-phenylprop-2-enyl)ammonium bromides in the presence of catalytic amounts of aqueous alkali do not undergo intramolecular [4+2]-cyclization of the diene synthesis type, because the 3-phenylprop-2-enyl group does not take part in the reaction as a diene fragment, and the initial salts are formed again. In the presence of a double amount of aqueous alkali, contrary to our expectations, the salts undergo Stevens rearrangement with transfer of the reaction center in both the host and migrating groups, followed by intramolecular cyclization rather than intramolecular cyclization–recyclization.
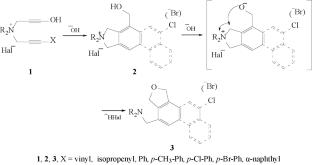
二烷基(4-羟基丁-2-炔基)(3-苯基丙-2-烯基)溴化铵在水碱存在下的重排-环化反应
摘要二烷基(4-羟基丁-2-炔基)(3-苯基丙-2-烯基)溴化铵在催化量的水碱存在下不会发生二烯合成类型的分子内[4+2]环化反应,因为 3-苯基丙-2-烯基不会作为二烯片段参与反应,并且会再次形成初始盐。与我们的预期相反,在双倍量的水碱存在下,盐类会发生史蒂文斯重排,宿主基团和迁移基团中的反应中心都发生转移,随后发生分子内环化,而不是分子内环化-再环化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


