The Multiple Student Conceptions of the Chemical Bond in a Quantum Chemical Context
IF 2.9
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The chemical bond is described with various context-sensitive models ranging from two electrons being shared between nuclei to the minimum-energy distance between the atoms. How we visualize these bonds further primes students to select one model over another. Previous research has targeted misconceptions of the chemical bond, but how they interact and evolve remains relatively underexplored. In this work, we examine the diversity of conceptions in bachelor’s chemistry students who have started to learn about quantum chemistry. We present results of a thematic analysis of data produced by bachelor students at different stages in their studies while interacting with a chemical simulation triangulated with a metaphor analysis of interviews with students taking a quantum chemistry lecture. We found that the chemical bond was largely conceptualized either as a rigid entity or in relation to energy in writing and informally metaphorically as community. We argue that while these conceptualizations are valid models, the students struggle to understand the contextual dependency and the plurality of different models.
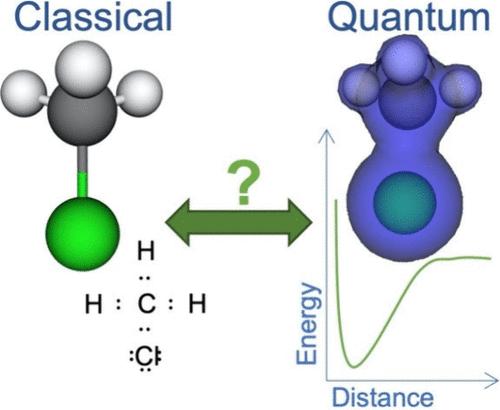
量子化学背景下学生对化学键的多重认识
化学键是通过各种与上下文相关的模型来描述的,从原子核之间共享两个电子到原子之间的最小能量距离,不一而足。我们如何将这些化学键形象化,进一步促使学生选择一种模式而不是另一种模式。以往的研究针对的是对化学键的错误认识,但对它们如何相互作用和演变的研究相对较少。在这项研究中,我们研究了已开始学习量子化学的化学学士学生的各种概念。我们对本科生在不同学习阶段与化学模拟互动时产生的数据进行了专题分析,并对参加量子化学讲座的学生的访谈进行了隐喻分析。我们发现,化学键在很大程度上被概念化为一个僵硬的实体,或在书面上与能量相关,或在非正式的隐喻中与共同体相关。我们认为,虽然这些概念都是有效的模型,但学生们很难理解语境依赖性和不同模型的多元性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


