Switching anti-EGFR antibody re-sensitizes head and neck cancer patient following acquired resistance to cetuximab
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
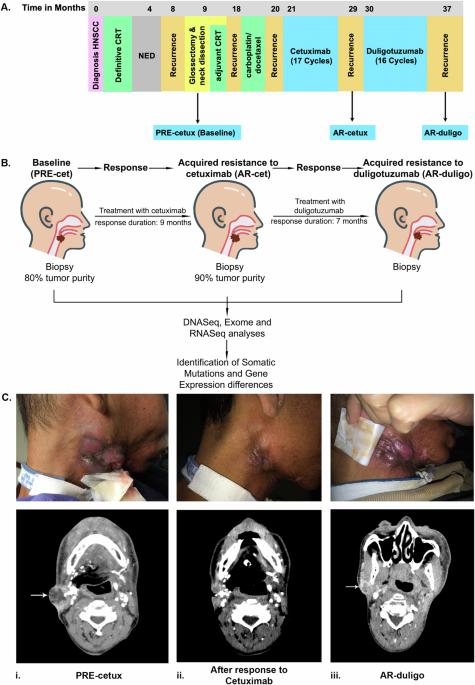
Cetuximab induces responses in about 13% of head and neck squamous cell carcinomas (HNSCC). We describe the molecular mechanism of acquired resistance to cetuximab, which could be overcome by switching to a different anti-EGFR antibody. Biopsies were collected at three different time points: before the start of cetuximab (PRE-cetux), at acquired resistance to cetuximab (AR-cetux), and at acquired resistance to duligotuzumab (AR-duligo). Biopsies were analyzed using tumor and normal whole-exome sequencing, RNASeq, and targeted panel sequencing with ultra-deep coverage to generate differential mutation and expression profiles. WES and targeted sequencing analysis identified an EGFR p.G465R extracellular domain mutation in AR-cetux biopsy. Furthermore, RNASeq confirmed the expression of this mutation in the tumor tissue. This mutation prevented the binding of cetuximab to EGFR and was not present in PRE-cetux and AR-duligo biopsies, suggesting a potential mechanism of acquired resistance to cetuximab. Molecular dynamic simulations confirmed that duligotuzumab effectively binds EGFR with a p.G465R mutation. Interestingly, the p.G465R mutation improved the stability of the duligotuzumab-EGFR complex as compared to the wild-type EGFR. This is the first report of an EGFR ECD mutation associated with acquired resistance to cetuximab, posing a need for further validation. We suggest appropriate serial mutational profiling to identify ECD mutations should be considered for select patients with initial cetuximab benefit.
转换抗表皮生长因子受体(EGFR)抗体可使对西妥昔单抗产生耐药性的头颈癌患者重新获得敏感性。
西妥昔单抗可诱导约13%的头颈部鳞状细胞癌(HNSCC)发生反应。我们描述了西妥昔单抗获得性耐药性的分子机制,这种耐药性可以通过换用不同的抗表皮生长因子受体(EGFR)抗体来克服。我们在三个不同的时间点采集了活检样本:西妥昔单抗起效前(PRE-cetux)、西妥昔单抗获得性耐药时(AR-cetux)以及杜利妥珠单抗获得性耐药时(AR-duligo)。使用肿瘤和正常人全外显子组测序、RNASeq和超深度覆盖的靶向面板测序对活检组织进行分析,以生成不同的突变和表达谱。WES和靶向测序分析在AR-cetux活检组织中发现了表皮生长因子受体p.G465R胞外结构域突变。此外,RNASeq也证实了这种突变在肿瘤组织中的表达。这种突变阻止了西妥昔单抗与表皮生长因子受体的结合,而且在PRE-西妥昔单抗和AR-duligo活检中不存在这种突变,这表明了西妥昔单抗获得性耐药的潜在机制。分子动力学模拟证实,杜利妥珠单抗能有效结合 p.G465R 突变的表皮生长因子受体。有趣的是,与野生型表皮生长因子受体(EGFR)相比,p.G465R 突变提高了杜利妥珠单抗-EGFR 复合物的稳定性。这是首次报道与西妥昔单抗获得性耐药性相关的表皮生长因子受体ECD突变,因此需要进一步验证。我们建议,应考虑对初始西妥昔单抗获益的部分患者进行适当的序列突变分析,以确定 ECD 突变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


