TOX2 nuclear-cytosol translocation is linked to leukemogenesis of acute T-cell leukemia by repressing TIM3 transcription
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
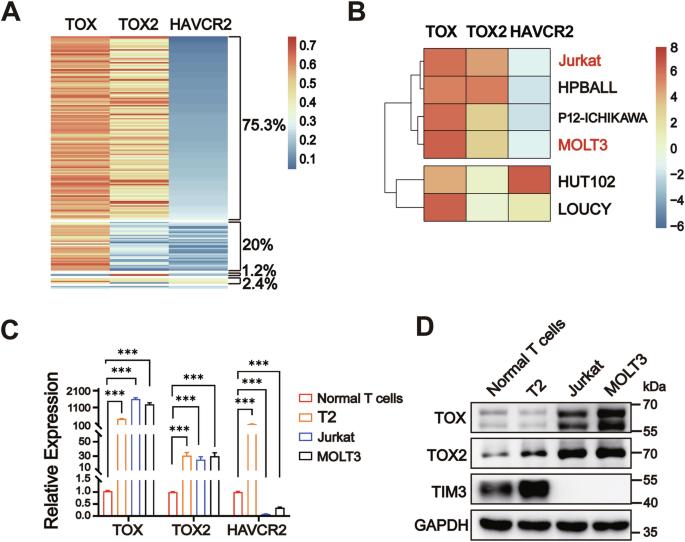
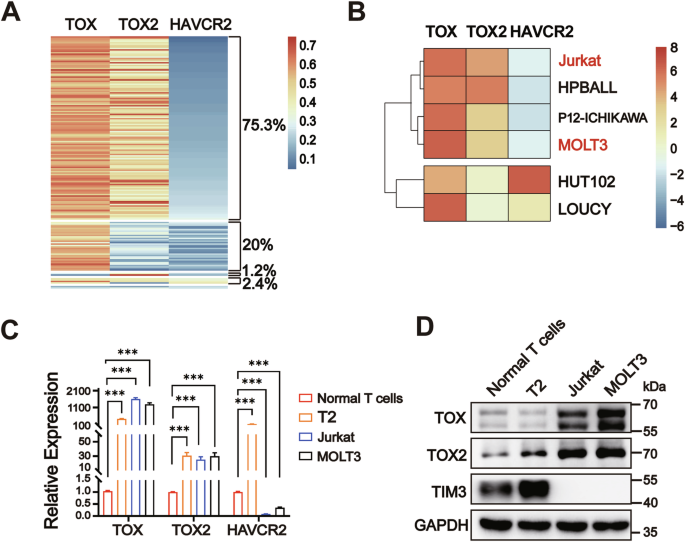
Nuclear factors TOX and TOX2 upregulate TIM3 expression and lead to T-cell exhaustion in malignancies. Here, we demonstrate two distinct TIM3 expression patterns (high & low) with high TOX and TOX2 levels in T-cell acute lymphoblastic leukemia (T-ALL) specimens and cell lines. However, the mechanisms regulated by TOX and TIM3 signaling in leukemogenesis are unclear. We found that TOX and TOX2 proteins each directly upregulated HAVCR2 transcription, while the cellular localization of TOX2 was different in Jurkat and MOLT3 cells (nucleus) and lymphoblastic cell T2 and normal T cells (cytoplasm). Nuclear TOX and TOX2 formed a protein complex and repressed HAVCR2 promoter activity by recruiting transcriptional corepressor LCOR and deacetylase HDAC3. The nuclear-cytosol translocation of TOX2 was deacetylation-dependent and cooperatively mediated by deacetylase Sirt1 and kinase TBK1. Radiation damage induced TOX2 nuclear translocation and decreased Sirt1, TIM3, and caspase 1 expression in normal T cells. Accordingly, knockdown of TOX, TOX2 or LCOR; HDAC3 inhibition; or TIM3 overexpression induced Jurkat cell apoptosis in vitro and slow growth in vivo. Thus, our findings demonstrate a novel regulatory mechanism involving TOX-TOX2 and the TIM3 pathway in the leukemogenesis of T-ALL.
TOX2核-胞浆转位通过抑制TIM3转录与急性T细胞白血病的白血病发生有关。
核因子 TOX 和 TOX2 能上调 TIM3 的表达,导致恶性肿瘤中 T 细胞衰竭。在这里,我们展示了T细胞急性淋巴细胞白血病(T-ALL)标本和细胞系中两种不同的TIM3表达模式(高和低),以及高水平的TOX和TOX2。然而,TOX 和 TIM3 信号在白血病发生过程中的调控机制尚不清楚。我们发现,TOX 和 TOX2 蛋白各自直接上调 HAVCR2 的转录,而 TOX2 在 Jurkat 和 MOLT3 细胞(细胞核)以及淋巴母细胞 T2 和正常 T 细胞(细胞质)中的细胞定位不同。核TOX和TOX2形成蛋白复合物,通过招募转录核心抑制因子LCOR和去乙酰化酶HDAC3抑制HAVCR2启动子的活性。TOX2的核-胞浆转位依赖于去乙酰化,并由去乙酰化酶Sirt1和激酶TBK1协同介导。辐射损伤诱导了TOX2的核转位,并降低了正常T细胞中Sirt1、TIM3和caspase 1的表达。因此,体外敲除 TOX、TOX2 或 LCOR;抑制 HDAC3 或 TIM3 过表达可诱导 Jurkat 细胞凋亡,并减缓体内生长。因此,我们的研究结果证明了TOX-TOX2和TIM3通路在T-ALL白血病发生过程中的新型调控机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


