Sacrificial Anode-Free Electrochemical Cross-Electrophile Coupling of 1,3-Diol Derivatives to Form Aliphatic and Aryl Cyclopropanes.
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Cross-electrophile coupling reactions that forge C(sp3)-C(sp3) bonds are strategic methods for the synthesis of molecules with high F(sp3), yet very few employ electrochemical conditions as the necessary reductant. Herein, we report an electrochemical intramolecular cross-electrophile coupling reaction of 1,3-diol derivatives to access aliphatic and aryl cyclopropanes, including spirocyclic and fused bicyclic cyclopropanes. The scalable electrochemical cross-electrophile coupling (eXEC) reaction employs a nonsacrificial anode in an undivided cell.
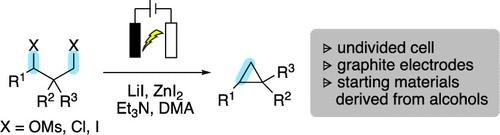
1,3-二醇衍生物的无牺牲阳极交叉亲电偶联形成脂肪族和芳基环丙烷。
形成 C(sp3)-C(sp3)键的交叉亲电偶联反应是合成高 F(sp3)分子的重要方法,但采用电化学条件作为必要还原剂的反应却寥寥无几。在此,我们报告了一种分子内 1,3-二醇衍生物的电化学交叉亲电偶联反应,以获得脂肪族和芳基环丙烷,包括螺环和融合双环环丙烷。这种可扩展的电化学交-亲电偶联(eXEC)反应在不分区电池中采用了非人工阳极。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


