Modifications to the NEATS instrument for more appropriate and reproducible assessment of guidelines trustworthiness
Abstract
Rational
The National Guideline Clearinghouse Extent of Adherence to Trustworthy Standards (NEATS) instrument measures the adherence of clinical practice guidelines to the trustworthiness standards proposed by the Institute of Medicine. However, rather than trustworthiness or methodological quality, the NEATS instrument evaluates the quality of reporting in addressing the management of conflict of interest of guideline development group members, systematic review process and assessment of the certainty of the evidence, rating the strength of recommendations, and updating plans. Furthermore, the NEATS instrument instructions on rating items are sufficiently limited that they may compromise the reproducibility of the instrument.
Methods (Modifications to the NEATS Instrument)
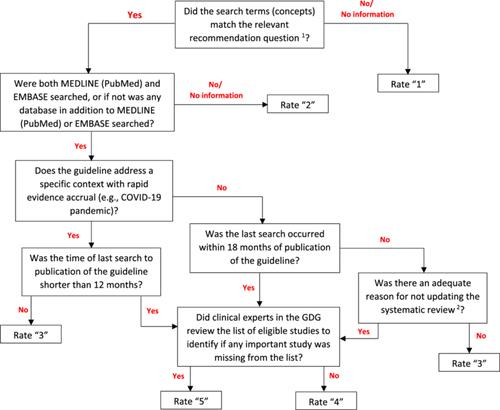
In the context of a specific methodological research project, we developed modifications to the NEATS instrument that include modifying the wording of items to capture trustworthiness rather than reporting quality and creating an algorithm for all items that maps responses to a series of prompting questions and guides the user in arriving at a rating from 1 to 5 or yes, no, or unknown, as applicable.
Implications
Modifications to the NEATS instrument present a structured and practical framework to assess the degree to which clinical practice guidelines are trustworthy, and they ensure that items evaluate trustworthiness and improve the reproducibility of assessments across a range of raters' level of expertise in guideline methodology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


