Transition metal-free approach for the synthesis of N-arylated piperidones and its ketals from ketenedithioacetal
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
N-Arylated piperidones were present as pharmacophores in many pharmaceuticals and useful precursors for the construction of new important molecules. We have developed a transition metal-free, cost-effective and mild approach for the synthesis of N-arylated/heteroarylated piperidones and its ketal by using ketal of piperidones and 2-oxo-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles as precursors. The desired product was achieved in 2 steps including amination of 2-oxo-5,6-dihydro-2H-benzo[h]chromene-3-carbonitriles from piperidone followed by ring transformation using a suitable nucleophile source. We have successfully tethered functionalized dihydrophenanthrenes, hydrobenzo[c]phenanthrenes and benzoquinolines at N-piperidinone under transition metal free conditions.
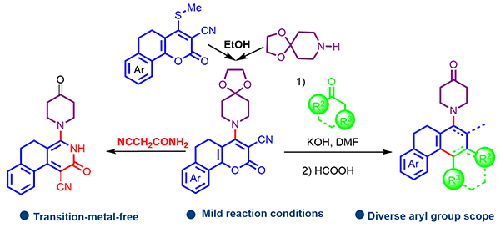
从二硫代乙醛合成 N-芳基哌啶酮及其酮类的无过渡金属方法
N-芳基化哌啶酮是许多药物的药源,也是构建新的重要分子的有用前体。我们以哌啶酮酮化物和 2-氧代-5,6-二氢-2H-苯并[h]色烯-3-甲腈为前体,开发了一种无过渡金属、低成本、温和的方法来合成 N-芳基化/异芳基化哌啶酮及其酮化物。我们分两步获得了所需产物,包括先将 2-氧代-5,6-二氢-2H-苯并[h]色烯-3-甲腈与哌啶酮胺化,然后使用合适的亲核剂进行环转化。在无过渡金属条件下,我们成功地在 N-哌啶酮上拴系了官能化的二氢菲、氢苯并[c]菲和苯并喹啉。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


