Nano-photosensitizers with gallic acid-involved Fe–O–Cu “electronic storage station” bridging ligand-to-metal charge transfer for efficient catalytic theranostics
Abstract
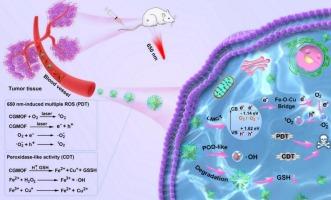
NH2-MIL-88B (Fe) (MOF) is a promising photocatalytic material for antitumor therapy because of its distinctive electronic structure. However, inadequate separation of photo-generated electrons and slow reaction rate in low/high-valence iron (Fe) cycles limit their clinical application. In the present study, “electronic storage station” as a ligand-to-metal charge transfer bridge bond was constructed to inhibit recombination of electron/hole under 650 nm laser irradiation. Cupric (Cu) ions and gallic acid (GA) were self-assembled into a MOF (denoted as CGMOF) to create an FeO(GA)
Cu bridge bond. GA, characterized by robust electron delocalization and abundant electron-donating groups, significantly enhances electron transfer efficiency for photodynamic therapy (PDT). CGMOF can respond to endogenous glutathione and release cuprous ions, accelerating the iron ion/ferrous ion cycles for chemodynamic therapy (CDT). The released Fe species can serve as T2-weighted magnetic resonance imaging contrast. Extended X-ray absorption fine structure spectra confirmed the presence of GA-containing Fe
O
Cu bonds in CGMOF. Furthermore, a series of photo-electrochemical tests confirmed that the formation of Fe
O(GA)
Cu bond prominently elevated the redox capacity and increased the carrier density of CGMOF by 2.74-fold compared to that of MOF. In addition, cinnamaldehyde was grafted onto CGMOF for tumor-responsive hydrogen peroxide self-supply. Concurrently, hyaluronic acid was surface-modified to achieve the targeted delivery of nano-photosensitizers. In summary, this study presents an innovative approach for engineering Fe-based metal–organic frameworks for synergetic PDT/CDT applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


