Applying a Guided Inquiry Approach to a Classic Practical on Chemoselective Reduction
IF 2.5
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
By adapting a well-established expository style practical on the topic of chemoselectivity, we present our successful approach to introducing elements of guided inquiry and decision-making to novice undergraduate chemists. Using nitroacetophenone as the polyfunctional substrate, students investigate the effect of using two different classes of reducing agents (reducing metal vs hydride donor). By changing the emphasis of the practical to be investigatory in nature, students actively deduce the outcomes of the different reactions, choosing suitable spectroscopic techniques to establish product identification. This enables students to take greater ownership of their learning, leading to the development of higher-level cognitive skills and improved engagement. While the originally reported NaBH4/EtOH reduction proceeds smoothly, significant issues encountered with product isolation using the Sn/HCl reduction conditions meant that a new alternative was required. We report here the use of Fe/NH4Cl as a superior reagent combination, maintaining complete chemoselectivity but providing numerous other benefits. Comparison between the two reducing metal reductions provides a useful scenario for discussion of sustainability in chemistry. Thin layer chromatography (TLC) reaction monitoring, including analysis of TLC-mass spectrometry data, are new facets to the investigative nature of this part of the practical and allowed students to postulate reduction to the hydroxylamine intermediate.
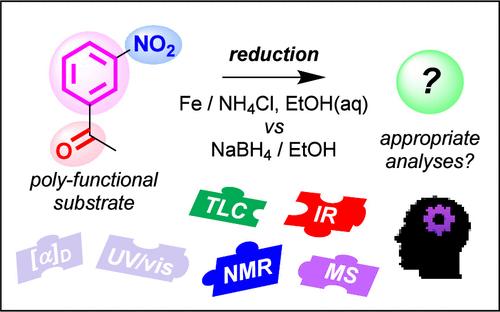
将引导式探究方法应用于化学选择性还原经典实践
通过对化学选择性这一主题上成熟的阐述式实用方法进行改编,我们介绍了向本科化学家新手介绍引导式探究和决策要素的成功方法。以硝基苯乙酮为多官能基质,学生们研究了使用两类不同还原剂(还原金属与氢化物供体)的效果。通过改变实践的重点,使其具有探究性质,学生积极推断不同反应的结果,选择合适的光谱技术来确定产物。这让学生能够更自主地学习,从而发展更高层次的认知技能,提高参与度。虽然最初报告的 NaBH4/EtOH 还原反应进展顺利,但使用 Sn/HCl 还原条件进行产物分离时遇到了重大问题,这意味着需要一种新的替代方法。我们在此报告使用 Fe/NH4Cl 作为一种更优越的试剂组合,不仅保持了完全的化学选择性,还带来了许多其他好处。这两种还原金属还原反应的比较为讨论化学的可持续性提供了一个有用的场景。薄层色谱(TLC)反应监测,包括 TLC-质谱数据分析,是这部分实践活动探究性质的新方面,并使学生能够推测出羟胺中间体的还原过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


