Modeling on in vivo disposition and cellular transportation of RNA lipid nanoparticles via quantum mechanics/physiologically-based pharmacokinetic approaches
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The lipid nanoparticle (LNP) has been so far proven as a strongly effective delivery system for mRNA and siRNA. However, the mechanisms of LNP's distribution, metabolism, and elimination are complicated, while the transportation and pharmacokinetics (PK) of LNP are just sparsely investigated and simply described. This study aimed to build a model for the transportation of RNA-LNP in Hela cells, rats, mice, and humans by physiologically based pharmacokinetic (PBPK) and quantum mechanics (QM) models with integrated multi-source data. LNPs with different ionizable lipids, particle sizes, and doses were modeled and compared by recognizing their critical parameters dominating PK. Some interesting results were found by the models. For example, the metabolism of ionizable lipids was first limited by the LNP disassembly rate instead of the hydrolyzation of ionizable lipids; the ability of RNA release from endosomes for three ionizable lipids was quantitively derived and can predict the probability of RNA release. Moreover, the biodegradability of three ionizable lipids was estimated by the QM method and the is generally consistent with the result of PBPK result. In summary, the transportation model of RNA LNP among various species for the first time was successfully constructed. Various in vitro and in vivo pieces of evidence were integrated through QM/PBPK multi-level modeling. The resulting new understandings are related to biodegradability, safety, and RNA release ability which are highly concerned issues of the formulation. This would benefit the design and research of RNA-LNP in the future.
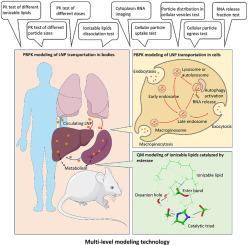
通过量子力学/生理学药代动力学方法建立 RNA 脂质纳米粒子体内处置和细胞运输模型
迄今为止,脂质纳米粒子(LNP)已被证明是一种非常有效的 mRNA 和 siRNA 运送系统。然而,LNP 的分布、代谢和消除机制十分复杂,而对其运输和药代动力学(PK)的研究和描述却很少。本研究旨在通过基于生理学的药代动力学(PBPK)和量子力学(QM)模型,整合多源数据,建立RNA-LNP在Hela细胞、大鼠、小鼠和人体内的转运模型。通过识别主导 PK 的关键参数,对不同可电离脂质、粒度和剂量的 LNPs 进行了建模和比较。模型得出了一些有趣的结果。例如,可电离脂质的代谢首先受限于LNP的分解率,而不是可电离脂质的水解率;对三种可电离脂质从内体释放RNA的能力进行了定量推导,并可预测RNA释放的概率。此外,用QM方法估算了三种可电离脂质的生物降解能力,结果与PBPK结果基本一致。综上所述,该研究首次成功构建了 RNA LNP 在不同物种间的运输模型。通过 QM/PBPK 多层次建模,整合了各种证据。由此产生的新认识涉及生物降解性、安全性和 RNA 释放能力等制剂中备受关注的问题。这将有利于未来 RNA-LNP 的设计和研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


