COLUMBIA-1: a randomised study of durvalumab plus oleclumab in combination with chemotherapy and bevacizumab in metastatic microsatellite-stable colorectal cancer
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
To determine whether the addition of durvalumab (anti-PD-L1) and oleclumab (anti-CD73) to standard-of-care treatment (FOLFOX and bevacizumab) enhances the anti-tumour effect in patients with metastatic colorectal cancer (mCRC). COLUMBIA-1 (NCT04068610) was a Phase Ib (feasibility; Part 1)/Phase II (randomised; Part 2) trial in patients with treatment-naïve microsatellite stable mCRC. Patients in Part 2 were randomised to receive standard-of-care (control arm) or standard-of-care plus durvalumab and oleclumab (experimental arm). Primary objectives included safety and efficacy. Seven patients were enrolled in Part 1 and 52 in Part 2 (n = 26 in each arm). Grade ≥3 treatment-emergent adverse events (TEAE) occurred in 80.8% and 65.4% of patients in the control and experimental arms of Part 2, respectively, with 26.9% and 46.3% experiencing serious TEAEs. The confirmed objective response rate (ORR) was numerically higher in the experimental arm compared with the control arm (61.5% [95% confidence interval (CI), 40.6–79.8] vs 46.2% [95% CI, 26.6–66.6]) but did not meet the statistically significant threshold in either arm. The safety profile of FOLFOX and bevacizumab in combination with durvalumab and oleclumab was manageable; however, the efficacy results do not warrant further development of this combination in patients with microsatellite stable mCRC. NCT04068610.
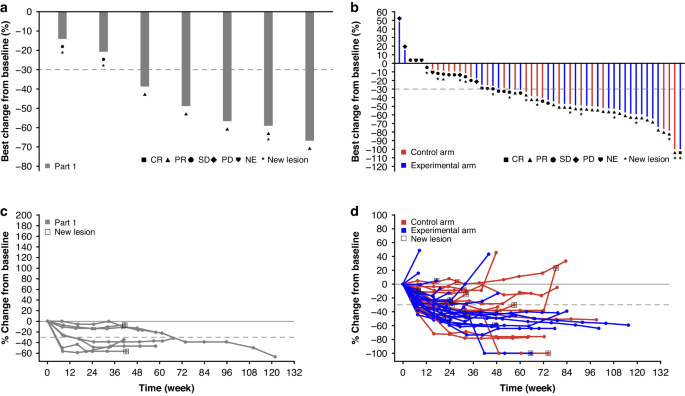

COLUMBIA-1:一项关于转移性微卫星稳定型结直肠癌中杜瓦单抗+奥利单抗联合化疗和贝伐单抗的随机研究。
研究背景目的:确定在标准治疗(FOLFOX和贝伐单抗)的基础上加用durvalumab(抗PD-L1)和oleclumab(抗CD73)是否能增强转移性结直肠癌(mCRC)患者的抗肿瘤效果:COLUMBIA-1(NCT04068610)是一项Ib期(可行性;第1部分)/II期(随机;第2部分)试验,针对微卫星稳定型mCRC治疗无效患者。第2部分的患者被随机分配接受标准治疗(对照组)或标准治疗加durvalumab和oleclumab(实验组)。主要目标包括安全性和有效性:第一部分有7名患者入组,第二部分有52名患者入组(每组26人)。在第2部分的对照组和实验组中,分别有80.8%和65.4%的患者发生了≥3级的治疗突发不良事件(TEAE),其中26.9%和46.3%的患者发生了严重的TEAE。与对照组相比,实验组的确诊客观反应率(ORR)在数字上更高(61.5% [95% 置信区间 (CI),40.6-79.8] vs 46.2% [95% CI,26.6-66.6]),但两组均未达到具有统计学意义的临界值:结论:FOLFOX和贝伐单抗与德瓦单抗和奥利珠单抗联合用药的安全性是可控的;但疗效结果并不支持在微卫星稳定的mCRC患者中进一步开发这种联合用药:注册:NCT04068610。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


