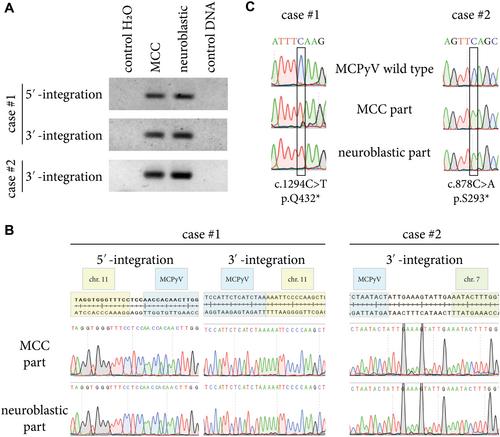
Analyses of combined Merkel cell carcinomas with neuroblastic components suggests that loss of T antigen expression in Merkel cell carcinoma may result in cell cycle arrest and neuroblastic transdifferentiation
IF 5.6
2区 医学
Q1 ONCOLOGY
Thibault Kervarrec, Silke Appenzeller, Susanne Gramlich, Etienne Coyaud, Kamel Bachiri, Romain Appay, Nicolas Macagno, Anne Tallet, Christine Bonenfant, Yannick Lecorre, Jean Kapfer, Sami Kettani, Nalini Srinivas, Kuan Cheok Lei, Anja Lange, Jürgen C Becker, Eva Maria Sarosi, Hervé Sartelet, Andreas von Deimling, Antoine Touzé, Serge Guyétant, Mahtab Samimi, David Schrama, Roland Houben
下载PDF
{"title":"Analyses of combined Merkel cell carcinomas with neuroblastic components suggests that loss of T antigen expression in Merkel cell carcinoma may result in cell cycle arrest and neuroblastic transdifferentiation","authors":"Thibault Kervarrec, Silke Appenzeller, Susanne Gramlich, Etienne Coyaud, Kamel Bachiri, Romain Appay, Nicolas Macagno, Anne Tallet, Christine Bonenfant, Yannick Lecorre, Jean Kapfer, Sami Kettani, Nalini Srinivas, Kuan Cheok Lei, Anja Lange, Jürgen C Becker, Eva Maria Sarosi, Hervé Sartelet, Andreas von Deimling, Antoine Touzé, Serge Guyétant, Mahtab Samimi, David Schrama, Roland Houben","doi":"10.1002/path.6304","DOIUrl":null,"url":null,"abstract":"<p>Merkel cell carcinoma (MCC) is an aggressive skin cancer frequently caused by genomic integration of the Merkel cell polyomavirus (MCPyV). MCPyV-negative cases often present as combined MCCs, which represent a distinctive subset of tumors characterized by association of an MCC with a second tumor component, mostly squamous cell carcinoma. Up to now, only exceptional cases of combined MCC with neuroblastic differentiation have been reported. Herein we describe two additional combined MCCs with neuroblastic differentiation and provide comprehensive morphologic, immunohistochemical, transcriptomic, genetic and epigenetic characterization of these tumors, which both arose in elderly men and appeared as an isolated inguinal adenopathy. Microscopic examination revealed biphasic tumors combining a poorly differentiated high-grade carcinoma with a poorly differentiated neuroblastic component lacking signs of proliferation. Immunohistochemical investigation revealed keratin 20 and MCPyV T antigen (TA) in the MCC parts, while neuroblastic differentiation was confirmed in the other component in both cases. A clonal relation of the two components can be deduced from 20 and 14 shared acquired point mutations detected by whole exome analysis in both combined tumors, respectively. Spatial transcriptomics demonstrated a lower expression of stem cell marker genes such as <i>SOX2</i> and <i>MCM2</i> in the neuroblastic component. Interestingly, although the neuroblastic part lacked TA expression, the same genomic MCPyV integration and the same large T-truncating mutations were observed in both tumor parts. Given that neuronal transdifferentiation upon TA repression has been reported for MCC cell lines, the most likely scenario for the two combined MCC/neuroblastic tumors is that neuroblastic transdifferentiation resulted from loss of TA expression in a subset of MCC cells. Indeed, DNA methylation profiling suggests an MCC-typical cellular origin for the combined MCC/neuroblastomas. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 1","pages":"112-124"},"PeriodicalIF":5.6000,"publicationDate":"2024-07-25","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6304","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6304","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Merkel cell carcinoma (MCC) is an aggressive skin cancer frequently caused by genomic integration of the Merkel cell polyomavirus (MCPyV). MCPyV-negative cases often present as combined MCCs, which represent a distinctive subset of tumors characterized by association of an MCC with a second tumor component, mostly squamous cell carcinoma. Up to now, only exceptional cases of combined MCC with neuroblastic differentiation have been reported. Herein we describe two additional combined MCCs with neuroblastic differentiation and provide comprehensive morphologic, immunohistochemical, transcriptomic, genetic and epigenetic characterization of these tumors, which both arose in elderly men and appeared as an isolated inguinal adenopathy. Microscopic examination revealed biphasic tumors combining a poorly differentiated high-grade carcinoma with a poorly differentiated neuroblastic component lacking signs of proliferation. Immunohistochemical investigation revealed keratin 20 and MCPyV T antigen (TA) in the MCC parts, while neuroblastic differentiation was confirmed in the other component in both cases. A clonal relation of the two components can be deduced from 20 and 14 shared acquired point mutations detected by whole exome analysis in both combined tumors, respectively. Spatial transcriptomics demonstrated a lower expression of stem cell marker genes such as SOX2 and MCM2 in the neuroblastic component. Interestingly, although the neuroblastic part lacked TA expression, the same genomic MCPyV integration and the same large T-truncating mutations were observed in both tumor parts. Given that neuronal transdifferentiation upon TA repression has been reported for MCC cell lines, the most likely scenario for the two combined MCC/neuroblastic tumors is that neuroblastic transdifferentiation resulted from loss of TA expression in a subset of MCC cells. Indeed, DNA methylation profiling suggests an MCC-typical cellular origin for the combined MCC/neuroblastomas. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
对含有神经母细胞成分的合并梅克尔细胞癌的分析表明,梅克尔细胞癌中 T 抗原表达的缺失可能会导致细胞周期停滞和神经母细胞的转分化。
梅克尔细胞癌(MCC)是一种侵袭性皮肤癌,常由梅克尔细胞多瘤病毒(MCPyV)基因组整合引起。MCPyV 阴性病例通常表现为合并 MCC,这是一个独特的肿瘤亚群,其特点是 MCC 与第二种肿瘤成分(主要是鳞状细胞癌)相关联。迄今为止,只有特殊病例报道过合并神经细胞分化的 MCC。在本文中,我们描述了另外两例伴有神经母细胞分化的合并 MCC,并对这些肿瘤进行了全面的形态学、免疫组织化学、转录组学、遗传学和表观遗传学特征描述,这两例肿瘤均发生于老年男性,表现为孤立的腹股沟腺病。显微镜检查发现,肿瘤呈双相,既有分化不良的高级别癌,也有分化不良的神经母细胞成分,但缺乏增殖迹象。免疫组化检查显示,MCC部分有角蛋白20和MCPyV T抗原(TA),而两个病例中的另一部分则被证实为神经母细胞分化。通过全外显子组分析,在两个合并肿瘤中分别发现了20个和14个共同的获得性点突变,由此可以推断这两个部分存在克隆关系。空间转录组学显示,神经母细胞部分的干细胞标志基因(如SOX2和MCM2)表达较低。有趣的是,虽然神经母细胞部分缺乏TA表达,但在两个肿瘤部分都观察到了相同的基因组MCPyV整合和相同的大T截断突变。鉴于有报道称 MCC 细胞系在 TA 受抑制时会发生神经元转分化,这两种 MCC/neuroblastic 合并肿瘤最有可能的情况是,神经母细胞转分化是由于 MCC 细胞中的一个亚群失去了 TA 的表达。事实上,DNA甲基化分析表明,MCC/神经母细胞瘤的合并细胞起源于MCC典型细胞。© 2024 作者。病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
The Journal of Pathology aims to serve as a translational bridge between basic biomedical science and clinical medicine with particular emphasis on, but not restricted to, tissue based studies. The main interests of the Journal lie in publishing studies that further our understanding the pathophysiological and pathogenetic mechanisms of human disease.
The Journal of Pathology welcomes investigative studies on human tissues, in vitro and in vivo experimental studies, and investigations based on animal models with a clear relevance to human disease, including transgenic systems.
As well as original research papers, the Journal seeks to provide rapid publication in a variety of other formats, including editorials, review articles, commentaries and perspectives and other features, both contributed and solicited.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


