Sustainable Brønsted-Lewis Acid Deep Eutectic Solvent for High Conversion of Rosin to Polymerized Rosin
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
The use of renewable rosin as a raw material to prepare rosin-based polymers with good properties had aroused great interest. This work used rosin as raw material and acid deep eutectic solvent [ZnCl2][FA]3 (synthesized by zinc chloride (ZnCl2) and formic acid (FA)) as a catalyst for high conversion of rosin to polymerized rosin. [ZnCl2][FA]3 possessed a suitable acid strength (457 mV), with both a Brønsted acid site and Lewis acid site, in which the Brønsted acid site could effectively promote the isomerization of rosin and the Lewis acid site could effectively promote the polymerization of rosin, so polymerized rosin with a high softening point (148.40 °C) and low acid value (125.23 mg KOH/g) was obtained under the following conditions: rosin = 15 g, mDES:mrosin = 30%, T = 110 °C, and t = 9 h. [ZnCl2][FA]3 maintained good catalytic performance in all 5 cycles; on this basis, the green synthesis technology of polymerized rosin under the DES system was given and not only promoted the further application of polymerized rosin but also realized the high value-added utilization of renewable resource rosin. In addition, the formation mechanism of DES was systematically explained through DFT calculation, and the reaction mechanism of rosin polymerization over [ZnCl2][FA]3 was explored, which could provide a theoretical basis for further research.
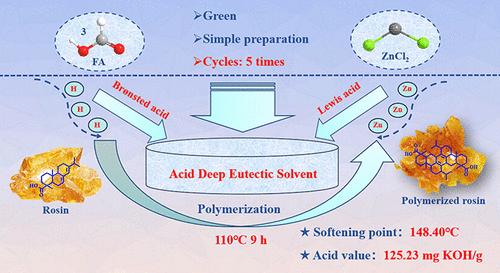
用于松香向聚合松香高转化率的可持续布氏-刘易斯酸深共晶溶剂
以可再生松香为原料制备具有良好性能的松香基聚合物引起了人们的极大兴趣。本研究以松香为原料,以酸性深共晶溶剂 [ZnCl2][FA]3(由氯化锌(ZnCl2)和甲酸(FA)合成)为催化剂,实现了松香到聚合松香的高转化率。[ZnCl2][FA]3 具有合适的酸度(457 mV),同时具有布氏酸位点和路易斯酸位点,其中布氏酸位点能有效促进松香的异构化,路易斯酸位点能有效促进松香的聚合,因此聚合松香具有较高的软化点(148.40 °C)和较低的酸值(125.在此基础上,给出了 DES 体系下聚合松香的绿色合成技术,不仅促进了聚合松香的进一步应用,而且实现了可再生资源松香的高附加值利用。此外,通过DFT计算系统地解释了DES的形成机理,探讨了松香在[ZnCl2][FA]3上聚合的反应机理,为进一步的研究提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


