HADHA promotes glioma progression by accelerating MDM2-mediated p53 ubiquitination
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
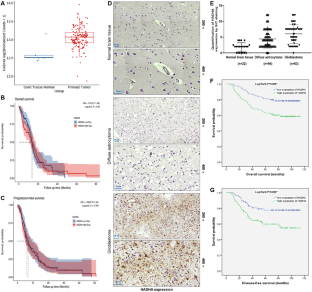
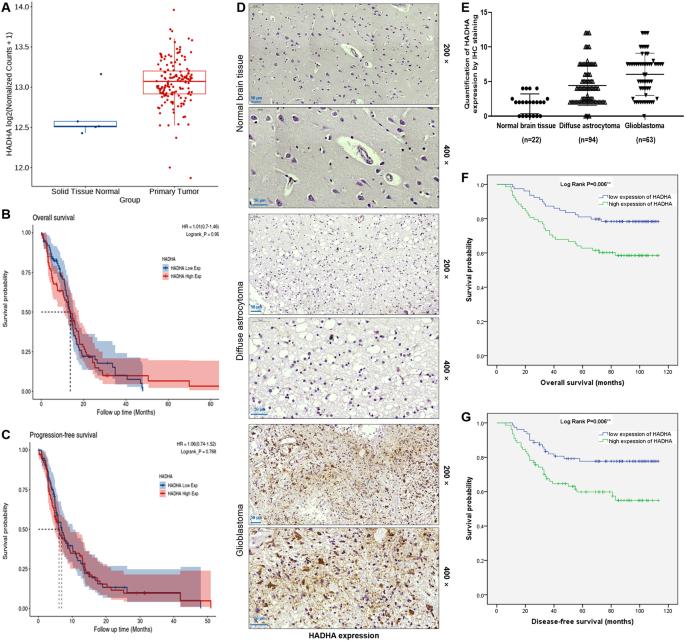
Glioma represents a notoriously aggressive and malignant tumor that targets the central nervous system, with a poor prognosis for patients. In this research, we set out to examine the role of hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha (HADHA) in glioma, its clinical significance, as well as its potential biological mechanisms. In this study, we used immunohistochemistry staining to assess the expression level of HADHA in glioma tissues. We also evaluated the correlation between HADHA expression and patient survival using the Kaplan–Meier method. To determine the role of HADHA in glioma cells, we conducted loss-of-function assays in vitro and in vivo. Additionally, we utilized co-immunoprecipitation and protein stability assays to investigate the potential mechanisms involving HADHA, MDM2, and p53 in glioma. Our research findings indicate that gliomas exhibit high levels of HADHA. Clinically, high expression of HADHA suggests an increased risk of malignant tumors, recurrence, and reduced survival rates. Functionally, knocking down HADHA can lead to decreased proliferation, enhanced apoptosis, and inhibited migration of glioma cells. Mechanistically, HADHA accelerates MDM2-mediated p53 ubiquitination through interaction with MDM2. Consistently, MDM2 knockdown or overexpression of p53 can attenuate the promoting effect of HADHA overexpression on the malignant progression of glioma. We have discovered a novel role of HADHA in promoting MDM2-mediated p53 ubiquitination, which contributes to the progression of glioma. This finding provides a new perspective to understand the pathogenesis of glioma and offers a potential target for developing innovative therapeutic strategies.
HADHA 通过加速 MDM2- 介导的 p53 泛素化促进胶质瘤进展
胶质瘤是一种以中枢神经系统为靶点的侵袭性恶性肿瘤,患者预后极差。本研究旨在探讨羟基乙酰-CoA脱氢酶三功能多酶复合物亚基α(HADHA)在胶质瘤中的作用、临床意义及其潜在的生物学机制。本研究采用免疫组化染色法评估了 HADHA 在胶质瘤组织中的表达水平。我们还采用 Kaplan-Meier 法评估了 HADHA 表达与患者生存期之间的相关性。为了确定 HADHA 在胶质瘤细胞中的作用,我们在体外和体内进行了功能缺失试验。此外,我们还利用共免疫共沉淀和蛋白质稳定性试验研究了脑胶质瘤中涉及HADHA、MDM2和p53的潜在机制。我们的研究结果表明,胶质瘤表现出高水平的 HADHA。在临床上,HADHA的高表达表明恶性肿瘤、复发和生存率降低的风险增加。在功能上,敲除 HADHA 可导致胶质瘤细胞增殖减少、凋亡增强和迁移受抑制。从机理上讲,HADHA通过与MDM2相互作用,加速了MDM2介导的p53泛素化。同样,MDM2敲除或过表达p53可以减弱HADHA过表达对胶质瘤恶性进展的促进作用。我们发现了HADHA在促进MDM2介导的p53泛素化中的新作用,而这种泛素化有助于胶质瘤的进展。这一发现为理解胶质瘤的发病机制提供了一个新的视角,并为开发创新治疗策略提供了一个潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


