Synthesis of 2-aminothiazoles containing 3-hydroxypyran-4-one fragment based on condensation of substituted α-arylaminoketones with thiourea
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Condensation of α-arylaminoketones bearing 3-hydroxypyran-4-one fragment with thiourea was studied. Based on considered investigation, the method for the synthesis of terarylenes with 2-aminothiazole bridge was elaborated. The presented process is the first example of construction of thiazole core starting from α-aminoketone precursor. The advantages of developed approach are easily available starting materials and convenient isolation of target products avoiding chromatographic purification. The structure of one of prepared products was confirmed by x-ray analysis. The synthetic utility of obtained 2-aminothiazoles with allomaltol substituent was demonstrated by reaction at amino and hydroxyl groups.
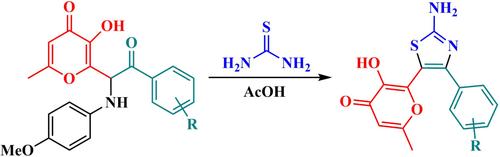
基于取代的 α-芳基氨基酮与硫脲的缩合合成含有 3- 羟基吡喃-4-酮片段的 2-氨基噻唑
研究了带有 3-hydroxypyran-4-one 片段的 α-arylaminoketones 与硫脲的缩合。在研究的基础上,详细阐述了 2-氨基噻唑桥合成萜烯的方法。所介绍的工艺是首个从 α-aminoketone 前体开始构建噻唑核心的实例。这种方法的优点是起始材料容易获得,目标产物的分离也很方便,无需色谱纯化。其中一种制备产物的结构已通过 X 射线分析得到证实。通过氨基和羟基反应,证明了所获得的带有异麦芽酮取代基的 2-氨基噻唑的合成用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


