The Role of Twist1 in Chronic Pancreatitis–Associated Pancreatic Stellate Cells
IF 4.7
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
In healthy pancreas, pancreatic stellate cells (PaSCs) synthesize the basement membrane, which is mainly composed of type IV collagen and laminin. In chronic pancreatitis (CP), PaSCs are responsible for the production of a rigid extracellular matrix (ECM) that is mainly composed of fibronectin and type I/III collagen. Reactive oxygen species evoke the formation of the rigid ECM by PaSCs. One source of reactive oxygen species is NADPH oxidase (Nox) enzymes. Nox1 up-regulates the expression of Twist1 and matrix metalloproteinase-9 (MMP-9) in PaSCs from mice with CP. This study determined the functional relationship between Twist1 and MMP-9, and other PaSC-produced proteins, and the extent to which Twist1 regulates digestion of ECM proteins in CP. Twist1 induced the expression of MMP-9 in mouse PaSCs. The action of Twist1 was not selective to MMP-9 because Twist1 induced the expression of types I and IV collagen, fibronectin, transforming growth factor, and α-smooth muscle actin. Luciferase assay indicated that Twist1 in human primary PaSCs increased the expression of MMP-9 at the transcriptional level in an NF-κB dependent manner. The digestion of type I/III collagen by MMP-9 secreted by PaSCs from mice with CP depended on Twist1. Thus, Twist1 in PaSCs from mice with CP induced rigid ECM production and MMP-9 transcription in an NF-κB–dependent mechanism that selectively displayed proteolytic activity toward type I/III collagen.
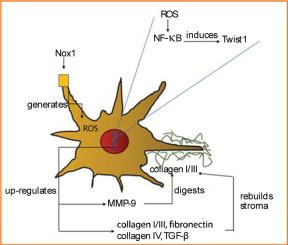
Twist1 在慢性胰腺炎相关胰腺星状细胞中的作用
在健康的胰腺中,胰腺星状细胞(PaSCs)合成基底膜,基底膜主要由胶原蛋白 IV 和层粘连蛋白组成。在慢性胰腺炎(CP)中,胰腺星状细胞负责制造刚性细胞外基质(ECM),该基质主要由纤维连接蛋白和胶原 I/III 组成。活性氧(ROS)可诱导肝细胞形成坚硬的细胞外基质(ECM)。ROS 的来源之一是 NADPH 氧化酶(Nox)。Nox1 能上调 CP 小鼠 PaSCs 中 Twist1 和基质金属蛋白酶(MMP)9 的表达。在此,我们确定了 1)Twist1 和 MMP-9 以及 PaSC 产生的其他蛋白质之间的功能关系;以及 2)Twist1 在多大程度上调节了 CP 中 ECM 蛋白的消化。Twist1 可诱导 mPaSCs 中 MMP-9 的表达。Twist1 的作用并不局限于 MMP-9,因为 Twist1 还能诱导胶原 I、胶原 IV、纤连蛋白、TGF-β 和 αSMA 的表达。利用荧光素酶试验,Twist1 在 hPaSCs 中以 NF-ĸB 依赖性方式在转录水平上增加了 MMP-9 的表达。CP 小鼠的 PaSCs 分泌的 MMP-9 对胶原 I/III 的消化依赖于 Twist1。因此,CP 小鼠 PaSCs 中的 Twist1 通过 NF-ĸB 依赖性机制诱导产生刚性 ECM 并转录 MMP-9,MMP-9 选择性地对胶原 I/III 具有蛋白水解活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


