Suppression of viral rebound by a Rev-dependent lentiviral particle in SIV-infected rhesus macaques
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
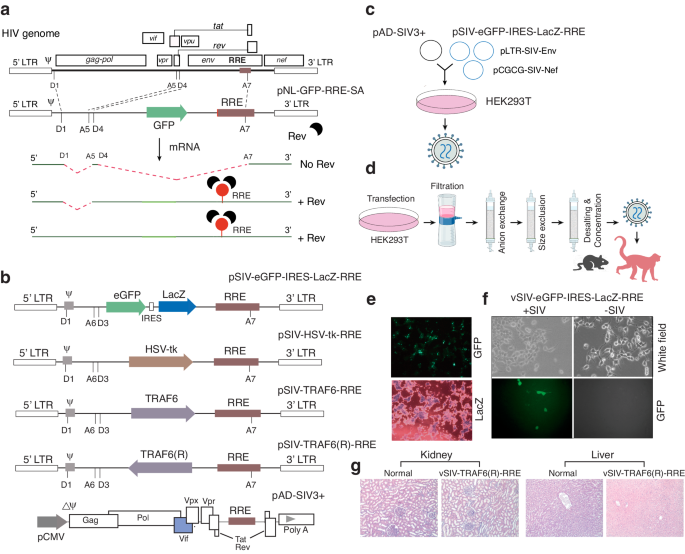
Persistence of human immunodeficiency virus (HIV) reservoirs prevents viral eradication, and consequently HIV-infected patients require lifetime treatment with antiretroviral therapy (ART) [1–5]. Currently, there are no effective therapeutics to prevent HIV rebound upon ART cessation. Here we describe an HIV/SIV Rev-dependent lentiviral particle that can be administered to inhibit viral rebound [6–9]. Using simian immunodeficiency virus (SIV)-infected rhesus macaques as a model, we demonstrate that the administration of pre-assembled SIV Rev-dependent lentiviral particles into SIVmac239-infected Indian rhesus macaques can lead to reduction of viral rebound upon ART termination. One of the injected animals, KC50, controlled plasma and CNS viremia to an undetectable level most of the time for over two years after ART termination. Surprisingly, detailed molecular and immunological characterization revealed that viremia control was concomitant with the induction of neutralizing antibodies (nAbs) following the administration of the Rev-dependent vectors. This study emphasizes the importance of neutralizing antibodies (nAbs) for viremia control [10–15], and also provides proof of concept that the Rev-dependent vector can be used to target viral reservoirs, including the CNS reservoirs, in vivo. However, future large-scale in vivo studies are needed to understand the potential mechanisms of viremia control induced by the Rev-dependent vector.

在感染 SIV 的猕猴体内,依赖 Rev 的慢病毒颗粒抑制了病毒反弹。
人类免疫缺陷病毒(HIV)储库的持续存在阻碍了病毒的根除,因此,HIV 感染者需要终生接受抗逆转录病毒疗法(ART)治疗 [1-5]。目前,还没有有效的疗法来防止抗逆转录病毒疗法停止后的艾滋病毒反弹。在此,我们描述了一种依赖于 HIV/SIV Rev 的慢病毒颗粒,这种颗粒可用于抑制病毒反弹 [6-9]。我们以感染了猿免疫缺陷病毒(SIV)的恒河猴为模型,证明了向感染了 SIVmac239 的印度恒河猴注射预组装的 SIV Rev 依赖性慢病毒颗粒可在抗逆转录病毒疗法终止时减少病毒反弹。其中一只名为 KC50 的注射动物在抗逆转录病毒疗法终止后的两年多时间里,血浆和中枢神经系统病毒血症大部分时间都控制在检测不到的水平。令人惊讶的是,详细的分子和免疫学特性分析表明,病毒血症的控制与依赖于 Rev 的载体的中和抗体(nAbs)的诱导同时发生。这项研究强调了中和抗体(nAbs)对病毒血症控制的重要性[10-15],同时也证明了依赖于Rev的载体可用于体内靶向病毒库(包括中枢神经系统病毒库)的概念。然而,未来还需要进行大规模的体内研究,以了解依赖 Rev 的载体诱导病毒血症控制的潜在机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


