Base-Promoted Reaction between N-Acyl Benzotriazoles and p-Toluenesulfonylmethyl Isocyanide (TosMIC): A Facile Synthesis of 4,5-Disubstituted Oxazoles
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We herein developed a base-promoted cyclization reaction between N-acyl benzotriazoles and p-toluenesulfonylmethyl isocyanide (TosMIC) to afford 4,5-disubstituted oxazoles. In the presence of 3 equiv of K3PO4, the two readily available starting materials reacted in N,N-dimethylformamide at 80 °C to give 28 examples of 4-tosyl-5-aryl, -alkyl, or -alkenyl-substituted oxazoles in moderate to high yields.
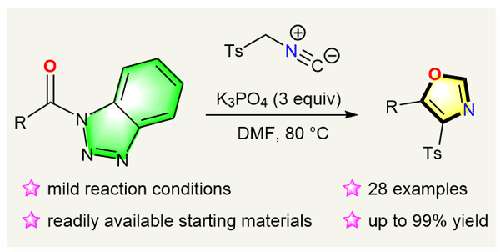
碱促进 N-酰基苯并三唑与对甲苯磺酰甲基异氰酸酯 (TosMIC) 的反应:4,5-二取代噁唑的简单合成
我们在此开发了一种 N-酰基苯并三唑与对甲苯磺酰甲基异氰酸酯 (TosMIC) 之间的碱促进环化反应,从而得到 4,5-二取代噁唑。在 3 等量 K3PO4 的存在下,两种易得的起始原料在 80 ℃ 的 N,N-二甲基甲酰胺中发生反应,以中等至高产率得到 28 个 4-对甲苯磺酰基-5-芳基、-烷基或-烯基取代的噁唑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


