Intravenous amoxicillin-clavulanic acid: prescribing practices in Australian hospitals
Abstract
Background
Oral amoxicillin-clavulanic acid, a broad-spectrum antimicrobial and one of the most commonly prescribed antimicrobials in Australia, has demonstrated poor prescribing guideline compliance and appropriateness. This may have inadvertent impacts on patient care and safety, from adverse drug reactions to antimicrobial resistance. Intravenous (IV) amoxicillin-clavulanic acid was first registered in Australia in 2017, reflecting a subsequent and immediate increase in use. There is a need to assess the quality of such prescribing.
Aim
To describe the quality of IV amoxicillin-clavulanic acid prescriptions through assessing guideline compliance and appropriateness of use in Australian hospitals.
Method
A retrospective data analysis of IV amoxicillin-clavulanic acid prescriptions from the Hospital National Antimicrobial Prescribing Survey database between 2013 and 2021. The main outcomes measured were guideline compliance and appropriateness. Ethical approval was granted by the Melbourne Health Human Research Ethics Committee (HREC/74195/MH-2022).
Results
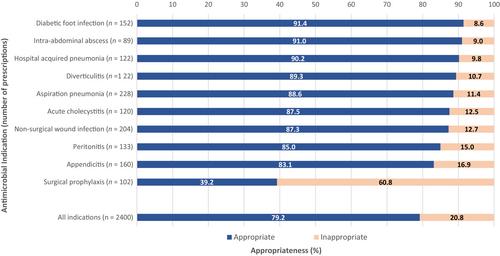
The proportion of prescriptions for IV amoxicillin-clavulanic acid, compared to all other antimicrobials, increased from 0.02% (2013) to 2.3% (2021). Guideline compliance and appropriateness have overall decreased (by 18.9% and 16.7%, respectively). Over time, national guidelines predominantly replaced local guidelines as the primary guideline source for prescribing. The most common reason for inappropriateness was unnecessarily broad spectrum of activity (39.5%).
Conclusion
Intravenous amoxicillin-clavulanic acid prescribing continues to increase throughout Australian hospitals, with notable reductions in guideline compliance and appropriateness. Since 2019, the increase in these outcomes coincided with updated national prescribing guidelines, evident by prescribers utilising national over local guidelines. These findings reinforce the concept that antimicrobial stewardship initiatives, including auditing, robust national guidelines and education, are crucial to optimise IV amoxicillin-clavulanic acid prescribing.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


