Development of new N-(4-((7-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazin-4-yl)oxy)-3-fluorophenyl)benzenesulfonamide analogues: Exploring anticancer potential via MerTK inhibition
引用次数: 0
Abstract
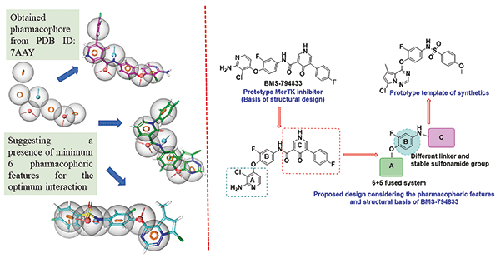
Mer proto-oncogene tyrosine-protein kinase (MerTK), a part of TAM (TYRO3, AXL and MerTK) family, is directly correlated with metastasis and various types of cancers. The inhibition of this receptor tyrosine kinase is one of the promising therapeutic strategies, as chemotherapy has higher efficacy. Considering the pharmacophoric features of the active domain of MerTK and structural characteristics of investigational drug, BMS794833, we have designed new N-(4-((7-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazin-4-yl)oxy)-3-fluorophenyl)benzenesulfonamide analogues (BDS-001 to BDS-005). In cytotoxic studies, BDS-001 displayed significantly higher cytotoxicity than cisplatin. It showed IC50 values of 2.09 µM, 1.96 µM and 3.08 µM against A549, MCF-7 and MDA-MB-231 cancer cell lines, respectively. In DMPK studies, BDS-001 was most stable and displayed moderate MerTK inhibitory potential. Molecular docking studies were performed to corroborate the MerTK inhibition, and BDS-001 was gave the most significant docking score (-12.33 Kcal/mol). Docking interactions demonstrated that imine and amine group of 3-chloropyridine moiety of BMS794833 formed a hydrogen bond with main chain of ATP pocket residue Met674, while oxygen atoms of 4-oxo-1,4-dihydropyridine-3-carboxamide moiety established hydrogen bonds with Lys619 and Asp741 amino acid residues of allosteric pocket of MerTK protein. These promising results are evident that N-(4-((7-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazin-4-yl)oxy)-3-fluorophenyl)benzenesulfonamide pharmacophore can provide potential insights in development of new MerTK inhibitors.
开发新的 N-(4-((7-氯-5-甲基吡咯并[2,1-f][1,2,4]三嗪-4-基)氧基)-3-氟苯基)苯磺酰胺类似物:通过抑制 MerTK 探索抗癌潜力
Mer原癌基因酪氨酸蛋白激酶(MerTK)是TAM(TYRO3、AXL和MerTK)家族的一部分,与癌症转移和各种癌症直接相关。抑制这种受体酪氨酸激酶是很有前景的治疗策略之一,因为化疗具有更高的疗效。考虑到 MerTK 活性域的药效特征和在研药物 BMS794833 的结构特点,我们设计了新的 N-(4-((7-氯-5-甲基吡咯并[2,1-f][1,2,4]三嗪-4-基)氧基)-3-氟苯基)苯磺酰胺类似物(BDS-001 至 BDS-005)。在细胞毒性研究中,BDS-001 的细胞毒性明显高于顺铂。它对 A549、MCF-7 和 MDA-MB-231 癌细胞株的 IC50 值分别为 2.09 µM、1.96 µM 和 3.08 µM。在DMPK研究中,BDS-001最为稳定,对MerTK的抑制潜力适中。分子对接研究证实了 BDS-001 对 MerTK 的抑制作用,其对接得分最高(-12.33 Kcal/mol)。对接相互作用表明,BMS794833 的 3-氯吡啶分子中的亚胺和胺基与 ATP 口袋残基 Met674 的主链形成了氢键,而 4-氧代-1,4-二氢吡啶-3-甲酰胺分子中的氧原子则与 MerTK 蛋白异构口袋中的 Lys619 和 Asp741 氨基酸残基建立了氢键。这些令人鼓舞的结果表明,N-(4-((7-氯-5-甲基吡咯并[2,1-f][1,2,4]三嗪-4-基)氧基)-3-氟苯基)苯磺酰胺药理结构可为开发新的 MerTK 抑制剂提供潜在的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


