“Fast but not so furious”: A condensed step-up dosing schedule of teclistamab for relapsed/refractory multiple myeloma
Abstract
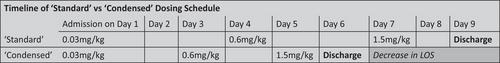
Teclistamab is a B-cell maturation antigen (BCMA)-directed bispecific T-cell engager approved for relapsed-refractory multiple myeloma (RRMM). Cytokine release syndrome (CRS) and Immune effector cell-associated neurotoxicity syndrome (ICANS) are well-documented treatment -related adverse events of teclistamab. The prescribing information recommends step-up dosing on days 1, 4, and 7 with 48–72 h of inpatient observation after each dose to monitor for CRS. This leads to a more than weeklong hospital stay, adding to the cost of therapy, resource utilization, and patient inconvenience. Here, we present a single center retrospective analysis addressing the safety and utility of a condensed step-up dosing schedule for teclistamab. All patients who were treated with teclistamab from November 2022 to August 2023 at the Medical University of South Carolina were included in the analysis. Patients received subcutaneous (SC) teclistamab with step-up doses (0.06 and 0.3 mg/kg) separated by either 2 or 3 (48–72 h) before the administration of the first full (1.5 mg/kg) dose (days 1, 3, and 5 ‘condensed’ schedule or days 1, 4, and 7 ‘standard’ schedule, respectively). All patients were hospitalized for the two step-up doses and first full dose of teclistamab and received pre-medications prior to each dose. Patients could be discharged after a minimum of 24 h following the full dose, if they did not have any CRS or ICANS. Relevant data regarding incidence, severity, and onset of CRS was collected. Statistical analysis was completed to assess the probability of fever with the first full dose of teclistamab based on incidence of fever with previous doses. A total of 25 patients were included in the analysis. Twenty-eight percent (7/25) of patients underwent the standard step up while the remaining 72% (18/25) underwent a condensed step up of teclistamab. More than half (53%, 13/25) of the patients experienced CRS during step up dosing. Grades 1 and 2 CRS occurred in 48% (12/25) and 4% (1/25) patients, respectively. Of the 13 patients that experienced CRS, 30% (4/13) fevered with the first dose, 84% (11/13) fevered with the second dose, and one patient developed fever after the third dose. The negative predictive value of being ‘fever free’ after doses 1 and 2 and remaining ‘fever free’ throughout hospitalization was 0.92. The median length of hospital stay among the 1, 3, and 5 step up group was 6 days (6–25) and 70% (14/20) of patients were discharged from the hospital within 7 days of treatment initiation. This report demonstrates the utility of a condensed step-up schedule for teclistamab initiation. The schedule was found to be safe and reduced hospital length of stay. These results should prompt consideration of shorter hospital stays for patients who do not experience CRS and raise the possibility of outpatient administration with close observation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


