Synthesis of N-Acyl-N’-Sulfonyl Hydrazides from Sulfonyl Hydrazides and Activated Amides
引用次数: 0
Abstract
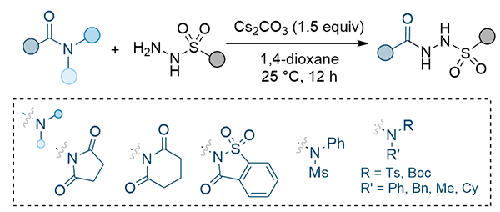
A methodology was developed for synthesizing acyl sulfonyl hydrazides through acyl substitution reactions between activated amides and arylsulfonyl hydrazides. Optimization of the reaction conditions revealed that using Cs₂CO₃ as a base and 1,4-dioxane as a solvent at 25 °C for 12 h produced the highest yields. Among various amides tested, N-benzoylsuccinimide was found to be the most reactive, with reduced reactivity observed for N-mesityl, N-tosyl and N-Boc substituted tertiary benzoyl amides. Cross-reactions between a diverse range of N-benzoylsuccinimides and arylsulfonyl hydrazides successfully produced the corresponding N-acyl-N’-sulfonyl hydrazides with yields ranging from 63% to 93%.
由磺酰基肼和活化酰胺合成 N-酰基-N'-磺酰基肼
通过活化酰胺和芳基磺酰肼之间的酰基取代反应,开发了一种合成酰基磺酰肼的方法。优化反应条件后发现,以 Cs₂CO₃为碱,1,4-二氧六环为溶剂,在 25 °C 下反应 12 小时的产率最高。在测试的各种酰胺中,发现 N-苯甲酰基琥珀酰亚胺的反应性最强,而 N-甲磺酰基、N-对甲磺酰基和 N-Boc 取代的叔苯甲酰基酰胺的反应性则较弱。各种 N-苯甲酰基琥珀酰亚胺与芳基磺酰肼之间的交叉反应成功地生成了相应的 N-酰基-N'-磺酰肼,产率为 63% 至 93%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


