Synthesis and Aggregation-Induced Emission Properties of New Carbazole-Based Pyranones and their Ring Transformed Derivatives
引用次数: 0
Abstract
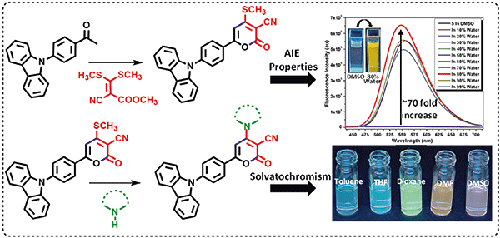
A new series of N-phenyl-carbazole (N-phCbz) appended pyranones were designed and synthesized using α-oxo-ketene-S, S-acetal under mild reaction conditions in good yields. The reactivity of donor-acceptor (D-A)-based 2H-pyranones was utilised to develop their ring-transformed benzene-cored N-phenyl-carbazole derivatives. All the synthesised carbazole-based pyranones showed aggregation-induced emission (AIE) characteristics in 80-99% water fraction (fw) in DMSO. Among all the synthesized compounds, 6-(4-(9H-carbazol-9-yl)phenyl)-4-(methylthio)-2-oxo-2H-pyran-3-carbonitrile exhibited excellent AIE behaviour with ~70 fold increase in fluorescence in 80% fw at 550nm. Furthermore, this compound showed exceptionally high fluorescence in nonpolar solvent (THF) as compared to polar solvents like DMSO (~200-fold increase in fluorescence). The DFT, DSC and TGA analysis of the synthesised D--A compounds suggested the strong electron donor ability of N-phCbz, with good thermal stability in the range of 231-393 °C. These N-phenyl-carbazole-appended pyranones with interesting AIE properties have great potential as probes for bioimaging applications as well as for optoelectronic materials.
新型咔唑基吡喃酮及其环变衍生物的合成与聚合诱导发射特性
在温和的反应条件下,利用α-氧代酮-S,S-缩醛设计并合成了一系列新的 N-苯基咔唑(N-phCbz)附加吡喃酮,产量良好。利用基于供体-受体(D-A)的 2H-吡喃酮的反应活性,开发出了它们的环状转化苯芯 N-苯基咔唑衍生物。所有合成的咔唑基吡喃酮在二甲基亚砜(DMSO)中 80-99% 的水组分(fw)中都显示出聚集诱导发射(AIE)特性。在所有合成的化合物中,6-(4-(9H-咔唑-9-基)苯基)-4-(甲硫基)-2-氧代-2H-吡喃-3-甲腈表现出了极佳的 AIE 性能,在 550nm 波长下,80% fw 的荧光增加了约 70 倍。此外,与 DMSO 等极性溶剂相比,该化合物在非极性溶剂(四氢呋喃)中的荧光特别高(荧光增加了约 200 倍)。对合成的 D--A 化合物进行的 DFT、DSC 和 TGA 分析表明,N-phCbz 具有很强的电子供体能力,在 231-393 °C 范围内具有良好的热稳定性。这些具有有趣的 AIE 特性的 N-苯基咔唑添加吡喃酮类化合物在生物成像应用和光电材料探针方面具有巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


