Design and synthesis of dexmedetomidine capped silver nanoparticles: Investigation of its catalytic application for reduction of nitrobenzenes and its effects on anesthetic, anti-inflammatory, antioxidant and analgesic applications
Abstract
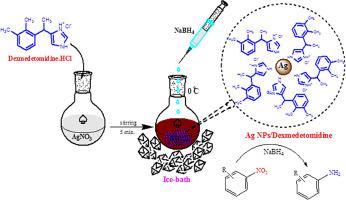
In the present work, the mono-dispersed produced dexmedetomidine capped silver nanoparticles were described. Dexmedetomidine-stabilized silver nanoparticles were prepared from the facile reduction of silver nitrate by NaBH4 as a reducing agent and dexmedetomidine as a stabilizing agent. The successful reduction of Ag+ ions into Ag0 NPs was confirmed by a visual formation of a deep brown color solution and the appearance of λmax nearing 450 nm in the UV–Vis study. The desired Ag NPs/Dexmedetomidine nanocomposite were characterized by various techniques such as UV/Vis, SEM-EDX, TEM and XRD. The abundant amino groups of dexmedetomidine provided the uniform dispersion and nano spherical shape of silver with sizes of 30–40 nm. The Ag NPs/Dexmedetomidine nanocomposite was applied in the catalytic reduction of diverse aromatic nitro functions using sodium borohydride as reducing agent. An excellent yield were achieved within very short reaction time. During the in vivo study, it was noted that the combination of Ag NPs/Dexmedetomidine showed local anesthetic properties in frog and guinea pig models. Additionally, oral administration of Ag NPs/Dexmedetomidine led to a notable decrease in paw edema in mice. The convulsive episodes triggered by acetic acid were markedly decreased in a dose-dependent manner in every group that was administered Ag NPs/Dexmedetomidine treatment. Additionally, the pain-relieving and anti-inflammatory properties of Ag NPs/Dexmedetomidine were assessed at different dosage levels. The data of this research provide evidence for the Ag NPs/Dexmedetomidine potential use as a pain-relieving and anesthetic substances in future investigations and medical trials involving human participants. In the antioxidant assessment, Ag NPs/Dexmedetomidine demonstrated IC50 values of 77 µg/mL against DPPH free radicals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


