Kv1.3-induced hyperpolarization is required for efficient Kaposi’s sarcoma–associated herpesvirus lytic replication
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
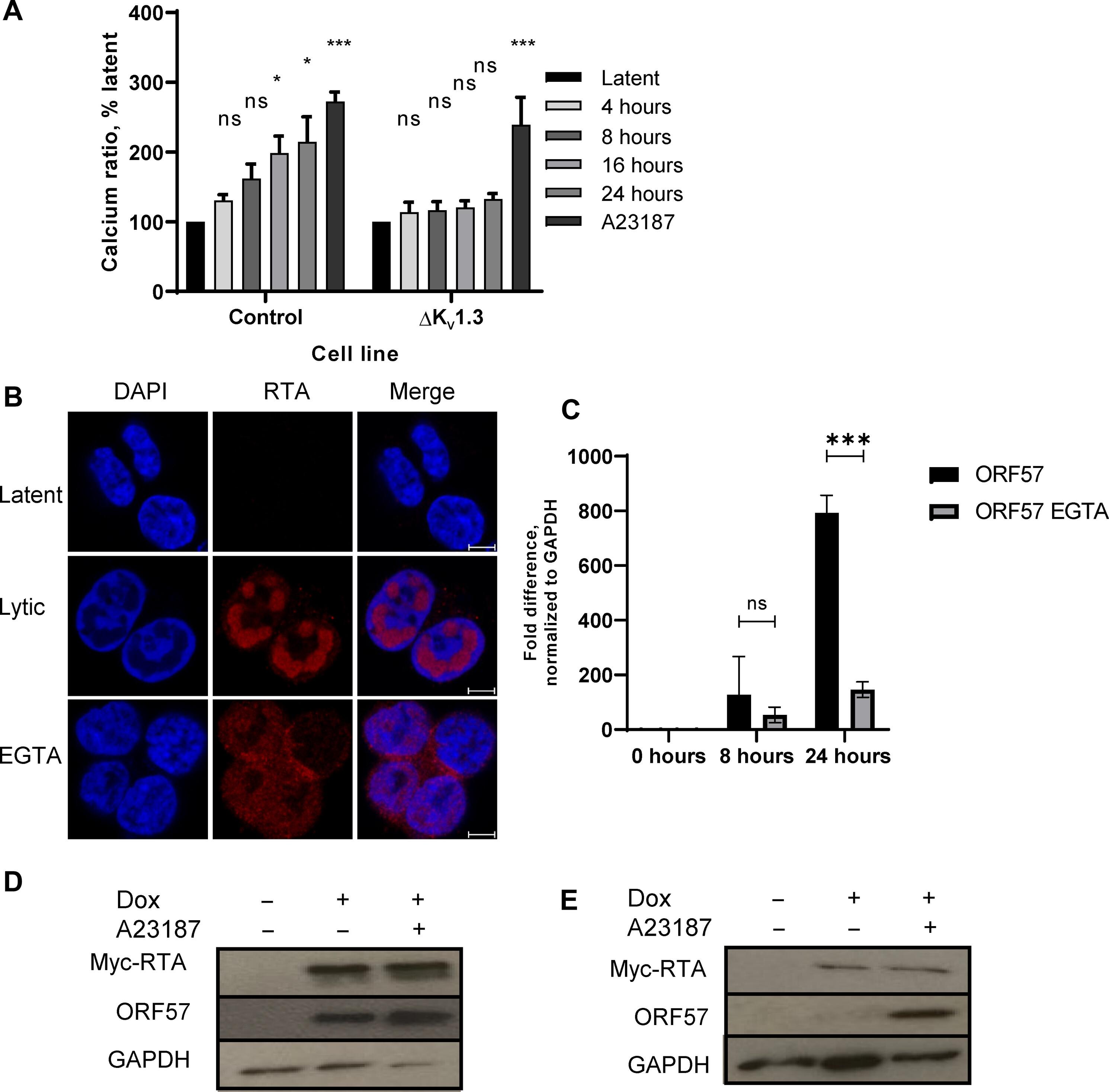
Kaposi’s sarcoma–associated herpesvirus (KSHV) is an oncogenic herpesvirus that is linked directly to the development of Kaposi’s sarcoma. KSHV establishes a latent infection in B cells, which can be reactivated to initiate lytic replication, producing infectious virions. Using pharmacological and genetic silencing approaches, we showed that the voltage-gated K+ channel Kv1.3 in B cells enhanced KSHV lytic replication. The KSHV replication and transcription activator (RTA) protein increased the abundance of Kv1.3 and led to enhanced K+ channel activity and hyperpolarization of the B cell membrane. Enhanced Kv1.3 activity promoted intracellular Ca2+ influx, leading to the Ca2+-driven nuclear localization of KSHV RTA and host nuclear factor of activated T cells (NFAT) proteins and subsequently increased the expression of NFAT1 target genes. KSHV lytic replication and infectious virion production were inhibited by Kv1.3 blockers or silencing. These findings highlight Kv1.3 as a druggable host factor that is key to the successful completion of KSHV lytic replication.
Kv1.3诱导的超极化是卡波西肉瘤相关疱疹病毒高效溶解复制所必需的。
卡波西肉瘤相关疱疹病毒(KSHV)是一种致癌疱疹病毒,与卡波西肉瘤的发病直接相关。KSHV 在 B 细胞中建立潜伏感染,可被重新激活以启动溶解复制,产生传染性病毒。通过药理学和基因沉默方法,我们发现 B 细胞中的电压门控 K+ 通道 Kv1.3 能增强 KSHV 的溶解复制。KSHV复制和转录激活剂(RTA)蛋白增加了Kv1.3的丰度,导致K+通道活性增强和B细胞膜超极化。增强的 Kv1.3 活性促进了细胞内 Ca2+ 的流入,导致 Ca2+ 驱动的 KSHV RTA 和宿主活化 T 细胞核因子(NFAT)蛋白的核定位,随后增加了 NFAT1 靶基因的表达。Kv1.3 阻断剂或沉默能抑制 KSHV 的溶解复制和传染性病毒的产生。这些发现突出表明,Kv1.3 是一种可药物治疗的宿主因子,是 KSHV 成功完成溶解复制的关键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


