Ubiquitin-specific protease 1 facilitates hepatocellular carcinoma progression by modulating mitochondrial fission and metabolic reprogramming via cyclin-dependent kinase 5 stabilization
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
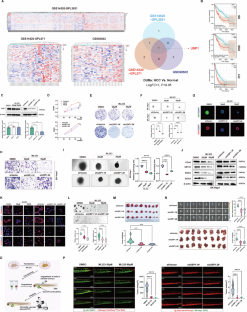
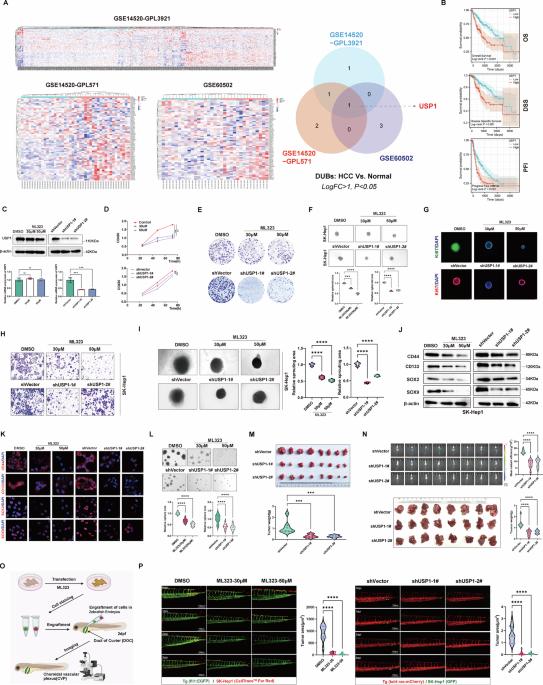
Although deubiquitinases (DUBs) have been well described in liver tumorigenesis, their potential roles and mechanisms have not been fully understood. In this study, we identified ubiquitin-specific protease 1 (USP1) as an oncogene with essential roles during hepatocellular carcinoma (HCC) progression. USP1, with elevated expression levels and clinical significance, was identified as a hub DUB for HCC in multiple bioinformatics datasets. Functionally, USP1 overexpression significantly enhanced the malignant behaviors in HCC cell lines and spheroids in vitro, as well as the zebrafish model and the xenograft model in vivo. In contrast, genetic ablation or pharmacological inhibition of USP1 dramatically impaired the phenotypes of HCC cells. Specifically, ectopic USP1 enhanced aggressive properties and metabolic reprogramming of HCC cells by modulating mitochondrial dynamics. Mechanistically, USP1 induced mitochondrial fission by enhancing phosphorylation of Drp1 at Ser616 via deubiquitination and stabilization of cyclin-dependent kinase 5 (CDK5), which could be degraded by the E3 ligase NEDD4L. The USP1/CDK5 modulatory axis was activated in HCC tissues, which was correlated with poor prognosis of HCC patients. Furthermore, Prasugrel was identified as a candidate USP1 inhibitor for targeting the phenotypes of HCC by an extensive computational study combined with experimental validations. Taken together, USP1 induced malignant phenotypes and metabolic reprogramming by modulating mitochondrial dynamics in a CDK5-mediated Drp1 phosphorylation manner, thereby deteriorating HCC progression.
泛素特异性蛋白酶1通过稳定细胞周期蛋白依赖性激酶5调节线粒体分裂和代谢重编程,从而促进肝细胞癌的发展。
虽然去泛素蛋白酶(DUBs)在肝脏肿瘤发生中的作用已被充分描述,但它们的潜在作用和机制尚未完全清楚。在这项研究中,我们发现泛素特异性蛋白酶 1(USP1)是一种在肝细胞癌(HCC)进展过程中发挥重要作用的癌基因。在多个生物信息学数据集中,表达水平升高且具有临床意义的 USP1 被确定为 HCC 的枢纽 DUB。从功能上讲,USP1 在体外过表达会显著增强 HCC 细胞系和球形细胞的恶性行为,在体内过表达也会增强斑马鱼模型和异种移植模型的恶性行为。相比之下,基因消融或药物抑制 USP1 会极大地损害 HCC 细胞的表型。具体来说,异位 USP1 通过调节线粒体动力学增强了 HCC 细胞的侵袭性和代谢重编程。从机制上讲,USP1通过去泛素化和稳定细胞周期蛋白依赖性激酶5(CDK5)来增强Drp1在Ser616处的磷酸化,从而诱导线粒体分裂,而CDK5可被E3连接酶NEDD4L降解。USP1/CDK5 调节轴在 HCC 组织中被激活,这与 HCC 患者的不良预后相关。此外,通过广泛的计算研究和实验验证,普拉格雷被确定为针对 HCC 表型的候选 USP1 抑制剂。总而言之,USP1通过CDK5介导的Drp1磷酸化方式调节线粒体动力学,诱导恶性表型和代谢重编程,从而恶化HCC进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


