GSDME promotes MASLD by regulating pyroptosis, Drp1 citrullination-dependent mitochondrial dynamic, and energy balance in intestine and liver
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
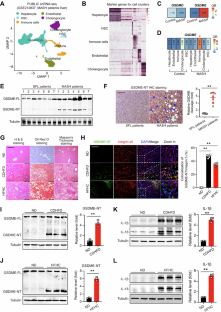
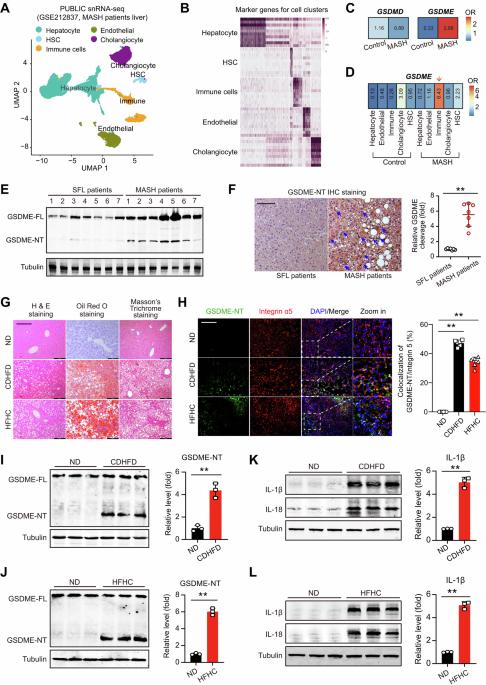
Dysregulated metabolism, cell death, and inflammation contribute to the development of metabolic dysfunction-associated steatohepatitis (MASH). Pyroptosis, a recently identified form of programmed cell death, is closely linked to inflammation. However, the precise role of pyroptosis, particularly gasdermin-E (GSDME), in MASH development remains unknown. In this study, we observed GSDME cleavage and GSDME-associated interleukin-1β (IL-1β)/IL-18 induction in liver tissues of MASH patients and MASH mouse models induced by a choline-deficient high-fat diet (CDHFD) or a high-fat/high-cholesterol diet (HFHC). Compared with wild-type mice, global GSDME knockout mice exhibited reduced liver steatosis, steatohepatitis, fibrosis, endoplasmic reticulum stress, lipotoxicity and mitochondrial dysfunction in CDHFD- or HFHC-induced MASH models. Moreover, GSDME knockout resulted in increased energy expenditure, inhibited intestinal nutrient absorption, and reduced body weight. In the mice with GSDME deficiency, reintroduction of GSDME in myeloid cells—rather than hepatocytes—mimicked the MASH pathologies and metabolic dysfunctions, as well as the changes in the formation of neutrophil extracellular traps and hepatic macrophage/monocyte subclusters. These subclusters included shifts in Tim4+ or CD163+ resident Kupffer cells, Ly6Chi pro-inflammatory monocytes, and Ly6CloCCR2loCX3CR1hi patrolling monocytes. Integrated analyses of RNA sequencing and quantitative proteomics revealed a significant GSDME-dependent reduction in citrullination at the arginine-114 (R114) site of dynamin-related protein 1 (Drp1) during MASH. Mutation of Drp1 at R114 reduced its stability, impaired its ability to redistribute to mitochondria and regulate mitophagy, and ultimately promoted its degradation under MASH stress. GSDME deficiency reversed the de-citrullination of Drp1R114, preserved Drp1 stability, and enhanced mitochondrial function. Our study highlights the role of GSDME in promoting MASH through regulating pyroptosis, Drp1 citrullination-dependent mitochondrial function, and energy balance in the intestine and liver, and suggests that GSDME may be a potential therapeutic target for managing MASH.
GSDME通过调节肠道和肝脏中的热蛋白沉积、Drp1瓜氨酸化依赖性线粒体动态和能量平衡,促进MASLD。
代谢失调、细胞死亡和炎症是代谢功能障碍相关性脂肪性肝炎(MASH)发病的原因。热蛋白沉积是最近发现的一种程序性细胞死亡形式,与炎症密切相关。然而,热蛋白沉积,尤其是气蛋白-E(GSDME)在 MASH 发展过程中的确切作用仍不清楚。在这项研究中,我们观察了 MASH 患者肝组织中的 GSDME 裂解和与 GSDME 相关的白细胞介素-1β(IL-1β)/IL-18 诱导,以及胆碱缺乏高脂饮食(CDHFD)或高脂/高胆固醇饮食(HFHC)诱导的 MASH 小鼠模型。与野生型小鼠相比,在CDHFD或HFHC诱导的MASH模型中,全基因组GSDME基因敲除小鼠的肝脏脂肪变性、脂肪性肝炎、纤维化、内质网应激、脂肪毒性和线粒体功能障碍均有所减轻。此外,GSDME基因敲除会导致能量消耗增加、肠道营养吸收受抑制以及体重下降。在缺乏GSDME的小鼠中,在髓细胞而不是肝细胞中重新引入GSDME可模拟MASH病理和代谢功能障碍,以及中性粒细胞胞外陷阱和肝巨噬细胞/单核细胞亚簇形成的变化。这些亚群包括Tim4+或CD163+常驻Kupffer细胞、Ly6Chi促炎单核细胞和Ly6CloCCR2loCX3CR1hi巡逻单核细胞的变化。RNA 测序和定量蛋白质组学的综合分析表明,在 MASH 期间,Dynamin 相关蛋白 1(Drp1)精氨酸-114(R114)位点的瓜氨酸化显著减少,这与 GSDME 有关。R114位点的Drp1突变降低了其稳定性,削弱了其重新分布到线粒体和调节有丝分裂的能力,并最终促进了其在MASH胁迫下的降解。GSDME 的缺乏逆转了 Drp1R114 的去瓜氨酸化,保持了 Drp1 的稳定性,并增强了线粒体的功能。我们的研究强调了GSDME通过调节肠道和肝脏中的热蛋白沉积、依赖于Drp1瓜氨酸化的线粒体功能以及能量平衡来促进MASH的作用,并表明GSDME可能是控制MASH的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


