Spatial metabolomics highlights metabolic reprogramming in acute myeloid leukemia mice through creatine pathway
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Acute myeloid leukemia (AML) is recognized as an aggressive cancer that is characterized by significant metabolic reprogramming. Here, we applied spatial metabolomics to achieve high-throughput, in situ identification of metabolites within the liver metastases of AML mice. Alterations at metabolite and protein levels were further mapped out and validated by integrating untargeted metabolomics and proteomics. This study showed a downregulation in arginine's contribution to polyamine biosynthesis and urea cycle, coupled with an upregulation of the creatine metabolism. The upregulation of creatine synthetases Gatm and Gamt, as well as the creatine transporter Slc6a8, resulted in a marked accumulation of creatine within tumor foci. This process further enhances oxidative phosphorylation and glycolysis of leukemia cells, thereby boosting ATP production to foster proliferation and infiltration. Importantly, we discovered that inhibiting Slc6a8 can counter these detrimental effects, offering a new strategy for treating AML by targeting metabolic pathways.
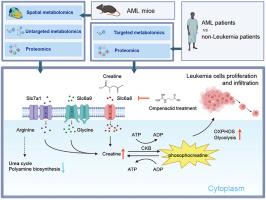
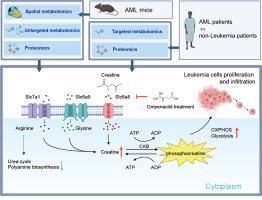
空间代谢组学凸显急性髓性白血病小鼠通过肌酸途径进行的代谢重编程
急性髓性白血病(AML)被认为是一种侵袭性癌症,其特征是显著的代谢重编程。在这里,我们应用空间代谢组学实现了对急性髓性白血病小鼠肝转移灶内代谢物的高通量原位鉴定。通过整合非靶向代谢组学和蛋白质组学,进一步绘制并验证了代谢物和蛋白质水平的变化。这项研究显示,精氨酸对多胺生物合成和尿素循环的贡献下调,同时肌酸代谢上调。肌酸合成酶 Gatm 和 Gamt 以及肌酸转运体 Slc6a8 的上调导致肌酸在肿瘤病灶内明显积累。这一过程进一步增强了白血病细胞的氧化磷酸化和糖酵解,从而提高了 ATP 的产生,促进了细胞的增殖和浸润。重要的是,我们发现抑制 Slc6a8 可以抵消这些有害影响,从而为通过靶向代谢途径治疗急性髓细胞白血病提供了一种新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


