Dysregulated Wnt/β-catenin signaling confers resistance to cuproptosis in cancer cells
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
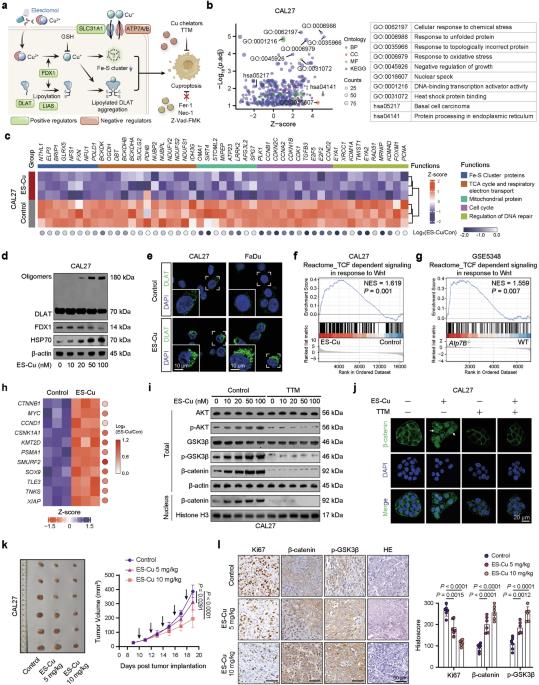
Cuproptosis is characterized by the aggregation of lipoylated enzymes of the tricarboxylic acid cycle and subsequent loss of iron-sulfur cluster proteins as a unique copper-dependent form of regulated cell death. As dysregulation of copper homeostasis can induce cuproptosis, there is emerging interest in exploiting cuproptosis for cancer therapy. However, the molecular drivers of cancer cell evasion of cuproptosis were previously undefined. Here, we found that cuproptosis activates the Wnt/β-catenin pathway. Mechanistically, copper binds PDK1 and promotes its interaction with AKT, resulting in activation of the Wnt/β-catenin pathway and cancer stem cell (CSC) properties. Notably, aberrant activation of Wnt/β-catenin signaling conferred resistance of CSCs to cuproptosis. Further studies showed the β-catenin/TCF4 transcriptional complex directly binds the ATP7B promoter, inducing its expression. ATP7B effluxes copper ions, reducing intracellular copper and inhibiting cuproptosis. Knockdown of TCF4 or pharmacological Wnt/β-catenin blockade increased the sensitivity of CSCs to elesclomol-Cu-induced cuproptosis. These findings reveal a link between copper homeostasis regulated by the Wnt/β-catenin pathway and cuproptosis sensitivity, and suggest a precision medicine strategy for cancer treatment through selective cuproptosis induction.
失调的 Wnt/β-catenin 信号传递使癌细胞对杯突症产生抗药性
杯突症的特点是三羧酸循环中脂酰化酶的聚集和随后铁硫簇蛋白质的丢失,是一种独特的依赖铜的细胞死亡调节形式。由于铜平衡失调可诱发杯突症,人们开始关注利用杯突症治疗癌症。然而,癌细胞逃避铜中毒的分子驱动因素此前尚未明确。在这里,我们发现杯突激活了 Wnt/β-catenin 通路。从机理上讲,铜与PDK1结合并促进其与AKT的相互作用,从而激活Wnt/β-catenin通路和癌症干细胞(CSC)特性。值得注意的是,Wnt/β-catenin 信号的异常激活赋予了癌干细胞对杯突变的抵抗力。进一步的研究表明,β-catenin/TCF4转录复合物直接结合ATP7B启动子,诱导其表达。ATP7B 可外流铜离子,减少细胞内铜含量,抑制杯突形成。敲除TCF4或药物阻断Wnt/β-catenin会增加CSCs对伊利司莫尔-铜诱导的杯突症的敏感性。这些发现揭示了由Wnt/β-catenin通路调控的铜稳态与杯突症敏感性之间的联系,并提出了通过选择性诱导杯突症治疗癌症的精准医学策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


