Asymmetric synthesis of 1H-pyrazolo[3,4-b]pyridine analogues catalyzed by chiral-at-metal Rh(iii) complexes†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient asymmetric Friedel–Crafts-type alkylation/cyclization of 5-aminopyrazoles with α,β-unsaturated 2-acyl imidazoles has been developed, affording corresponding pyrazolo[3,4-b]pyridine analogues in 81–98% yield with 85–99% enantioselectivity. Remarkably, this protocol exhibits extraordinary advantages in terms of reactivity and enantioselectivity, given the fact that chiral Rh(III) complex at an amount as low as 0.05 mol% can promote the title reaction on the gram scale to afford the desired product, with excellent enantioselectivity (96% ee). Application of the methodology in the efficient synthesis of pyrazolo[3,4-b]pyridine via dehydrogenation is reported.
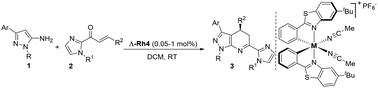
在手性金属 Rh(III) 配合物催化下不对称合成 1H-吡唑并[3,4-b]吡啶类似物
研究人员开发了一种高效的不对称弗里德尔-卡夫型烷基化/环化 5-氨基吡唑与 α,β-不饱和 2-酰基咪唑的方法,得到了相应的吡唑并[3,4-b] 吡啶类似物,收率为 81-98%,对映选择性为 85-99%。值得注意的是,由于低至 0.05 摩尔% 的手性 Rh(III) 复合物就能促进克级规模的标题反应,从而以出色的对映选择性(96% ee)得到所需的产物,因此该方法在反应性和对映选择性方面具有非凡的优势。报告还介绍了该方法在通过脱氢高效合成吡唑并[3,4-b] 吡啶中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


