Impact of coding risk variant IFNGR2 on the B cell-intrinsic IFN-γ signaling pathway in multiple sclerosis
IF 7.9
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
B cells of people with multiple sclerosis (MS) are more responsive to IFN-γ, corresponding to their brain-homing potential. We studied how a coding single nucleotide polymorphism (SNP) in IFNGR2 (rs9808753) co-operates with Epstein-Barr virus (EBV) infection as MS risk factors to affect the IFN-γ signaling pathway in human B cells. In both cell lines and primary cells, EBV infection positively associated with IFN-γ receptor expression and STAT1 phosphorylation. The IFNGR2 risk SNP selectively promoted downstream signaling via STAT1, particularly in transitional B cells. Altogether, EBV and the IFNGR2 risk SNP independently amplify IFN-γ signaling, potentially driving B cells to enter the MS brain.
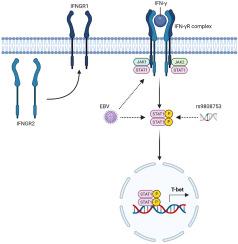
编码风险变体 IFNGR2 对多发性硬化症中 B 细胞内在 IFN-γ 信号通路的影响。
多发性硬化症(MS)患者的 B 细胞对 IFN-γ 的反应更加敏感,这与其大脑归属潜能相对应。我们研究了 IFNGR2(rs9808753)中的编码单核苷酸多态性(SNP)如何与作为多发性硬化症风险因素的爱泼斯坦-巴氏病毒(EBV)感染共同影响人类 B 细胞中的 IFN-γ 信号通路。在细胞系和原代细胞中,EBV 感染与 IFN-γ 受体表达和 STAT1 磷酸化呈正相关。IFNGR2 风险 SNP 可选择性地通过 STAT1 促进下游信号转导,尤其是在过渡性 B 细胞中。总之,EBV和IFNGR2风险SNP可独立地放大IFN-γ信号传导,从而可能促使B细胞进入多发性硬化症大脑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of autoimmunity
医学-免疫学
CiteScore
27.90
自引率
1.60%
发文量
117
审稿时长
17 days
期刊介绍:
The Journal of Autoimmunity serves as the primary publication for research on various facets of autoimmunity. These include topics such as the mechanism of self-recognition, regulation of autoimmune responses, experimental autoimmune diseases, diagnostic tests for autoantibodies, as well as the epidemiology, pathophysiology, and treatment of autoimmune diseases. While the journal covers a wide range of subjects, it emphasizes papers exploring the genetic, molecular biology, and cellular aspects of the field.
The Journal of Translational Autoimmunity, on the other hand, is a subsidiary journal of the Journal of Autoimmunity. It focuses specifically on translating scientific discoveries in autoimmunity into clinical applications and practical solutions. By highlighting research that bridges the gap between basic science and clinical practice, the Journal of Translational Autoimmunity aims to advance the understanding and treatment of autoimmune diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


