Synthesis of Methyl 2-[Bis(benzylthio)phosphoryl]acetate as a Novel Horner–Wadsworth–Emmons-Type Reagent and Its Application to the Diastereodivergent Synthesis of (E)- and (Z)-α,β-Unsaturated Esters
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Methyl 2-[bis(benzylthio)phosphoryl]acetate has proven to be an efficient Horner–Wadsworth–Emmons (HWE)-type reagent for the diastereodivergent synthesis of (E)- and (Z)-α,β-unsaturated esters. Under the conditions of excess NaHMDS relative to the HWE-type reagent, the HWE-type reactions of methyl 2-[bis(benzylthio)phosphoryl]acetate with various aldehydes afforded the corresponding α,β-unsaturated esters in an E-selective manner in up to 100:0 E/Z ratio. However, when an excess of the HWE-type reagent was used relative to NaHMDS, the stereoselectivity of the HWE-type reactions was dramatically changed from E to Z, yielding an E/Z ratio of up to 2:98.
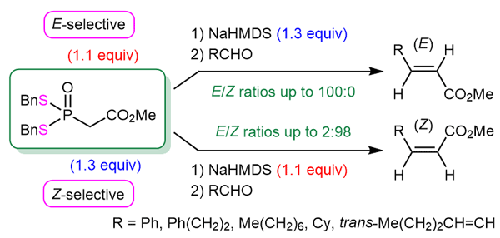
2-[Bis(benzylthio)phosphoryl]acetate 甲酯作为新型 Horner-Wadsworth-Emmons 型试剂的合成及其在 (E)- 和 (Z)-α,β- 不饱和酯的非对映异构合成中的应用
事实证明,2-[双(苄硫基)磷酰]乙酸甲酯是一种高效的 Horner-Wadsworth-Emmons (HWE) 型试剂,可用于 (E)- 和 (Z)-α,β- 不饱和酯的非对映异构合成。在 NaHMDS 相对于 HWE 型试剂过量的条件下,2-[双(苄硫基)磷酰]乙酸甲酯与各种醛的 HWE 型反应以 E 选择性方式得到相应的 α,β-不饱和酯,E/Z 比率高达 100:0。然而,当使用的 HWE 型试剂相对于 NaHMDS 过量时,HWE 型反应的立体选择性就会从 E 型急剧转变为 Z 型,产生的 E/Z 比率高达 2:98。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


