Cucurbitacins mitigate vascular neointimal hyperplasia by suppressing cyclin A2 expression and inhibiting VSMC proliferation
Abstract
Background
Restenosis frequently occurs after percutaneous angioplasty in patients with vascular occlusion and seriously threatens their health. Substantial evidence has revealed that preventing vascular smooth muscle cell proliferation using a drug-eluting stent is an effective approach to improve restenosis. Cucurbitacins have been demonstrated to exert an anti-proliferation effect in various tumors and a hypotensive effect. This study aims to investigate the role of cucurbitacins extracted from Cucumis melo L. (CuECs) and cucurbitacin B (CuB) on restenosis.
Methods
C57BL/6 mice were subjected to left carotid artery ligation and subcutaneously injected with CuECs or CuB for 4 weeks. Hematoxylin–Eosin, immunofluorescence and immunohistochemistry staining were used to evaluate the effect of CuECs and CuB on neointimal hyperplasia. Western blot, real-time PCR, flow cytometry analysis, EdU staining and cellular immunofluorescence assay were employed to measure the effects of CuECs and CuB on cell proliferation and the cell cycle in vitro. The potential interactions of CuECs with cyclin A2 were performed by molecular docking.
Results
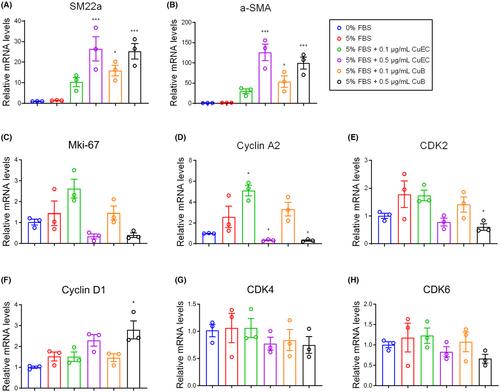
The results demonstrated that both CuECs and CuB exhibited significant inhibitory effects on neointimal hyperplasia and proliferation of vascular smooth muscle cells. Furthermore, CuECs and CuB mediated cell cycle arrest at the S phase. Autodocking analysis demonstrated that CuB, CuD, CuE and CuI had high binding energy for cyclin A2. Our study also showed that CuECs and CuB dramatically inhibited FBS-induced cyclin A2 expression. Moreover, the expression of cyclin A2 in CuEC- and CuB-treated neointima was downregulated.
Conclusions
CuECs, especially CuB, exert an anti-proliferation effect in VSMCs and may be potential drugs to prevent restenosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


