The cytoplasmic tail of myelin protein zero induces morphological changes in lipid membranes
Abstract
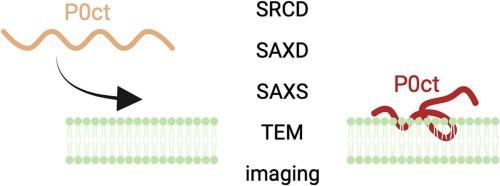
The major myelin protein expressed by the peripheral nervous system Schwann cells is protein zero (P0), which represents 50% of the total protein content in myelin. This 30-kDa integral membrane protein consists of an immunoglobulin (Ig)-like domain, a transmembrane helix, and a 69-residue C-terminal cytoplasmic tail (P0ct). The basic residues in P0ct contribute to the tight packing of myelin lipid bilayers, and alterations in the tail affect how P0 functions as an adhesion molecule necessary for the stability of compact myelin. Several neurodegenerative neuropathies are related to P0, including the more common Charcot-Marie-Tooth disease (CMT) and Dejerine-Sottas syndrome (DSS) as well as rare cases of motor and sensory polyneuropathy. We found that high P0ct concentrations affected the membrane properties of bicelles and induced a lamellar-to-inverted hexagonal phase transition, which caused bicelles to fuse into long, protein-containing filament-like structures. These structures likely reflect the formation of semicrystalline lipid domains with potential relevance for myelination. Not only is P0ct important for stacking lipid membranes, but time-lapse fluorescence microscopy also shows that it might affect membrane properties during myelination. We further describe recombinant production and low-resolution structural characterization of full-length human P0. Our findings shed light on P0ct effects on membrane properties, and with the successful purification of full-length P0, we have new tools to study the role of P0 in myelin formation and maintenance in vitro.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


