Merging Ring-Opening 1,2-Metallate Shift with Asymmetric C(sp3)–H Borylation of Aziridines
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Chiral secondary alkyl amines with a vicinal quaternary stereocenter are undoubtedly important and ubiquitous subunits in natural products and pharmaceuticals. However, their asymmetric synthesis remains a formidable challenge. Herein, we merge the ring-opening 1,2-metallate shift with iridium-catalyzed enantioselective C(sp3)–H borylation of aziridines to deliver these frameworks with high enantioselectivities. We also demonstrated the synthetic application by downstream transformations, including the total synthesis of two Amaryllidaceae alkaloids, (−)-crinane and (+)-mesmebrane.
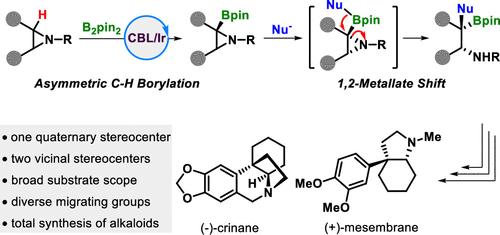
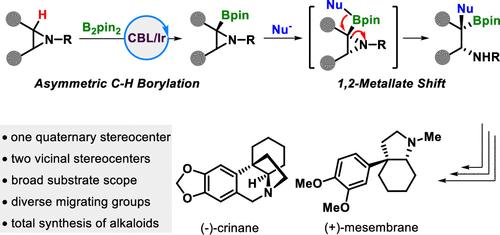
将氮丙啶的 1,2-金属环开环转变与不对称 C(sp3)-H Borylation 结合起来。
手性烷基仲胺具有一个侧季基立体中心,无疑是天然产物和药物中重要且无处不在的亚基。然而,它们的不对称合成仍然是一项艰巨的挑战。在本文中,我们将开环 1,2 金属酸盐转变与铱催化的氮丙啶对映选择性 C(sp3)-H 硼酰化结合在一起,以提供这些具有高对映选择性的框架。我们还通过下游转化展示了这一合成应用,包括两种金丝桃科生物碱--(-)-crinane 和 (+)-mesmebrane 的全合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


