Multiepitope recognition technology promotes the in-depth analysis of antibody‒drug conjugates
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The dynamic tracking of antibody‒drug conjugates (ADCs) in serum is crucial. However, a versatile bioanalytical platform is lacking due to serious matrix interferences, the heterogeneity and complex biotransformation of ADCs, and the recognition deficiencies of traditional affinity technologies. To overcome this, a multiepitope recognition technology (MERT) was developed by simultaneously immobilizing CDR and non-CDR ligands onto MOF@AuNPs. MERT's excellent specificity, ultrahigh ligand density, and potential synergistic recognition ability enable it to target the different key regions of ADCs to overcome the deficiencies of traditional technologies. The binding capacity of MERT for antibodies is ten to hundred times higher than that of the mono-epitope or Fc-specific affinity technologies. Since MERT can efficiently capture target ADCs from serum, a novel bioanalytical platform based on MERT and RPLC‒QTOF-MS has been developed to monitor the dynamic changes of ADCs in serum, including the fast changes of drug-to-antibody ratio from 3.67 to 0.22, the loss of payloads (maytansinol), and the unexpected hydrolysis of the succinimide ring of the linker, which will contribute to clarify the fate of ADCs and provide a theoretical basis for future design. In summary, the MERT-based versatile platform will open a new avenue for in-depth studies of ADCs in biological fluids.
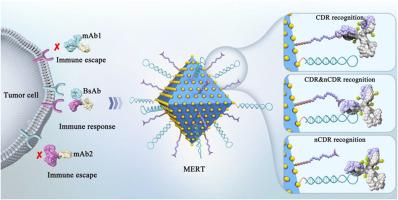
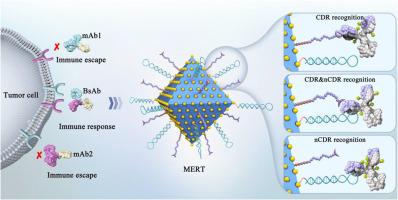
多表位识别技术促进了对抗体药物共轭物的深入分析
对血清中的抗体药物共轭物(ADC)进行动态跟踪至关重要。然而,由于基质干扰严重、ADC 的异质性和复杂的生物转化以及传统亲和技术的识别缺陷,目前还缺乏一个多功能的生物分析平台。为了克服这一问题,我们开发了一种多位点识别技术(MERT),将 CDR 和非 CDR 配体同时固定在 MOF@AuNPs 上。MERT 具有优异的特异性、超高的配体密度和潜在的协同识别能力,可针对 ADC 的不同关键区域进行识别,克服了传统技术的不足。MERT 与抗体的结合能力是单表位或 Fc 特异性亲和技术的十倍到百倍。由于 MERT 能从血清中有效捕获目标 ADC,因此开发了一种基于 MERT 和 RPLC-QTOF-MS 的新型生物分析平台,用于监测 ADC 在血清中的动态变化,包括药物与抗体比从 3.67 快速变为 0.22、有效载荷(maytansinol)的损失以及连接体琥珀酰亚胺环的意外水解,这将有助于阐明 ADC 的命运,并为未来的设计提供理论依据。总之,基于 MERT 的多功能平台将为深入研究生物液体中的 ADC 开辟一条新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
文献相关原料
公司名称
产品信息
阿拉丁
Hydroxypropyl methyl cellulose (HPMC)
阿拉丁
Hydrochloric acid
阿拉丁
Sodium hydroxide
阿拉丁
Sodium formate
阿拉丁
Sodium chloride
阿拉丁
Disodium hydrogen phosphate
阿拉丁
Monosodium phosphate
阿拉丁
Isopropanol
阿拉丁
Sodium citrate
阿拉丁
Chloroauric acid hydrated
阿拉丁
2-amino terephthalic acid
阿拉丁
Dimethyl formamide
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


