Electrosynthesis of Quinoxalines via Intermolecular Cyclization/Dehydrogenation of Ketones with o-Phenylenediamines
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
In this study, we proposed a novel electrochemical dehydrogenative synthetic method for preparing 2-substituted quinoxalines by intermolecular cyclization of aryl alkyl ketones and o-phenylenediamines. This method gave various quinoxalines in yields ranging from 35% to 71%. This novel protocol employs mild reaction conditions and offers moderate to excellent yields, a wide substrate scope, and broad functional-group compatibility. Furthermore, a late-stage functionalization and the wide substrate scope demonstrated the synthetic utility of this protocol.
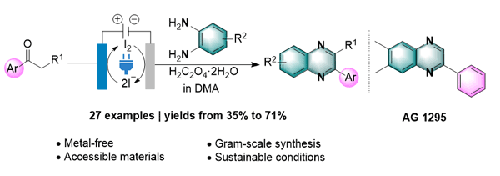
通过酮与邻苯二胺的分子间环化/脱氢反应电合成喹喔啉类化合物
在这项研究中,我们提出了一种新型的电化学脱氢合成方法,通过芳基烷基酮和邻苯二胺的分子间环化来制备 2-取代的喹喔啉类化合物。这种方法可以制备出各种喹喔啉类化合物,收率在 35% 到 71% 之间。这种新型方案采用温和的反应条件,具有中等到极好的产率、广泛的底物范围和广泛的官能团兼容性。此外,后期官能化和广泛的底物范围也证明了该方法的合成实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


