Tetraborylation of p-Benzynes Generated by the Masamune–Bergman Cyclization through Reaction Design Based on the Reaction Path Network
引用次数: 0
Abstract
Designing the reactant molecule of an initial reaction, based on quantum chemical pathway exploration, enabled us to access a new reaction, i.e., the tetraborylation reaction of p-benzynes generated from 1,2-diethynylbenzene derivatives, using bis(pinacolato)diborane(4) (B2pin2). Based on the reaction path network generated via the artificial-force-induced reaction (AFIR) method, desired and undesired paths were identified and used to modify the chemical structure of the reactant. After the in silico screening, the optimal structure of the reactant was determined to be a 1,2-diethynylbenzene derivative with a butylene linker. The reaction of the optimized reactant and its derivatives with an excess of B2pin2 gave the tetraborylated products in good yields (up to 58%). It is quite intriguing that the two carbons of p-benzyne behave formally as dicarbenes in this reaction.
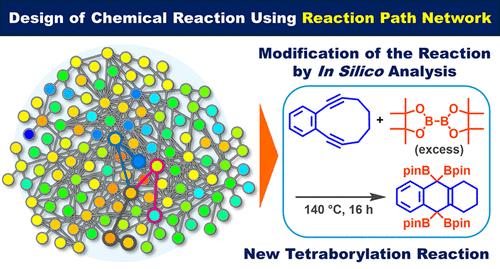
通过基于反应路径网络的反应设计对 Masamune-Bergman 环化反应生成的对苄基进行四乙酰化反应
在量子化学路径探索的基础上设计初始反应的反应物分子,使我们能够进入一个新的反应,即使用双(频哪醇胺)二硼烷(4) (B2pin2)对 1,2-二乙炔苯衍生物生成的对苄基炔进行四乙酰化反应。根据人工力诱导反应(AFIR)方法生成的反应路径网络,确定了需要和不需要的路径,并用于修改反应物的化学结构。经过硅学筛选,确定反应物的最佳结构为带有丁烯连接物的 1,2-二乙炔苯衍生物。将优化的反应物及其衍生物与过量的 B2pin2 反应,可以得到四溴化产物,而且收率很高(高达 58%)。耐人寻味的是,对苄的两个碳在这一反应中正式表现为二羰基。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


