Reduction of NOx on metal-free hydrogenated hexagonal boron nitride
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
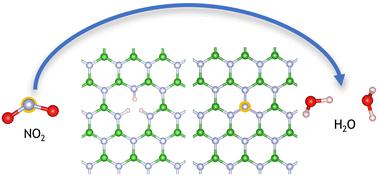
Sustainable catalysts are essential for critical industrial and environmental processes. 2D materials have exceptional surface area and unique thermal and electronic properties, making them excellent candidates for catalytic applications. Moreover, 2D materials can be functionalised to create metal-free active sites, which provide sustainable alternatives to transition and precious metals. Among the pollutants emitted by combustion engines, NOx stands out as one of the most detrimental gases, contributing to environmental pollution and posing risks to human health. We demonstrate that functionalised defects in hexagonal boron nitride (hBN) provide a thermodynamically viable route to removing NOx by reaction with a hydrogenated boron vacancy (3HVB). The decomposition of NO2 proceeds by initially overcoming an activation energy barrier of 1.12 eV to transfer a hydrogen atom from the surface, forming a NO2H species, followed by the elimination of a water molecule. A thermodynamically favourable product consisting of a surface-bound hydroxyl adjacent to a nitrogen antisite defect (where a nitrogen atom occupies a site typically occupied by a boron atom) forms after overcoming an energy barrier of 1.28 eV. NO can further decompose by overcoming an activation energy barrier of 2.23 eV to form a surface HNO species. A rearrangement of the HNO species takes place with an activation energy of 1.96 eV, followed by the elimination of water. The overall reactions reduce NOx into defective hBN and H2O.
在无金属氢化六方氮化硼上还原氮氧化物
可持续催化剂对于关键的工业和环境工艺至关重要。二维材料具有优异的表面积和独特的热学和电子特性,是催化应用的绝佳候选材料。此外,二维材料还可以功能化,形成无金属活性位点,为过渡金属和贵金属提供可持续的替代品。在内燃机排放的污染物中,氮氧化物是最有害的气体之一,不仅造成环境污染,还威胁人类健康。我们证明,六方氮化硼(hBN)中的功能化缺陷为通过与氢化硼空位(3HVB)反应去除氮氧化物提供了一条热力学上可行的途径。二氧化氮的分解首先要克服 1.12 eV 的活化能势垒,从表面转移一个氢原子,形成 NO2H 物种,然后消除一个水分子。在克服 1.28 eV 的能障后,会形成一种热力学上有利的产物,该产物由表面结合的羟基组成,邻近氮反位缺陷(氮原子占据了通常由硼原子占据的位置)。通过克服 2.23 eV 的活化能势垒,NO 可以进一步分解,形成表面 HNO 物种。HNO 物种的重排活化能为 1.96 eV,随后水被排出。整个反应将 NOx 还原成有缺陷的 hBN 和 H2O。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


