Biocompatible hydroxyapatite-based nano vehicle bypasses viral transduction and enables sustained silencing of a pluripotency marker gene, demonstrating desired differentiation in mouse embryonic stem cells
Abstract
Background
Differentiation of pluripotent stem cells into desired lineages is the key aspect of regenerative medicine and cell-based therapy. Although RNA interference (RNAi) technology is exploited extensively for this, methods for long term silencing of the target genes leading to differentiation remain a challenge. Sustained knockdown of the target gene by RNAi is often inefficient as a result of low delivery efficiencies, protocol induced toxicity and safety concerns related to viral vectors. Earlier, we established octa-arginine functionalized hydroxyapatite nano vehicles (R8HNPs) for delivery of small interfering RNA (siRNA) against a pluripotency marker gene in mouse embryonic stem cells. Although we demonstrated excellent knockdown efficiency of the target gene, sustained gene silencing leading to differentiation was yet to be achieved.
Methods
To establish a sustained non-viral gene silencing protocol using R8HNP, we investigated various methods of siRNA delivery: double delivery of adherent cells (Adh-D), suspension delivery followed by adherent delivery (Susp + Adh), single delivery in suspension (Susp-S) and multiple deliveries in suspension (Susp-R). Sustained knockdown of a pluripotent marker gene followed by differentiation was analysed by reverse transcriptase-PCR, fluoresence-activated cell sorting and immunofluorescence techniques. Impact on cell viability as a result of repeated exposure of the R8HNP was also tested.
Results
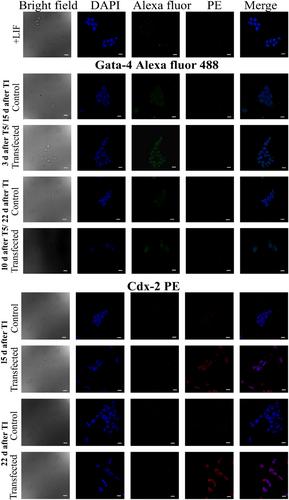
Amongst the protocols tested, the most efficient knockdown of the target gene for a prolonged period of time was obtained by repeated suspension delivery of the R8HNP-siRNA conjugate. The long-term silencing of a pluripotency marker gene resulted in differentiation of R1 ESCs predominantly towards the extra embryonic and ectodermal lineages. Cells displayed excellent tolerance to repeated exposures of R8HNPs.
Conclusions
The results demonstrate that R8HNPs are promising, biocompatible, non-viral alternatives for prolonged gene silencing and obtaining differentiated cells for therapeutics.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


