Tissue-resident memory T cells break tolerance to renal autoantigens and orchestrate immune-mediated nephritis
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
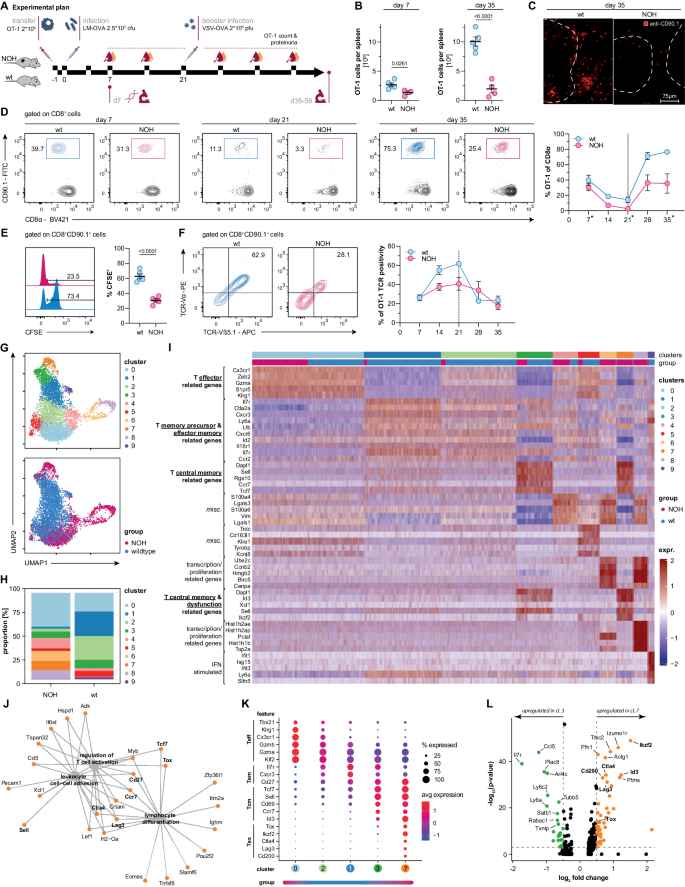
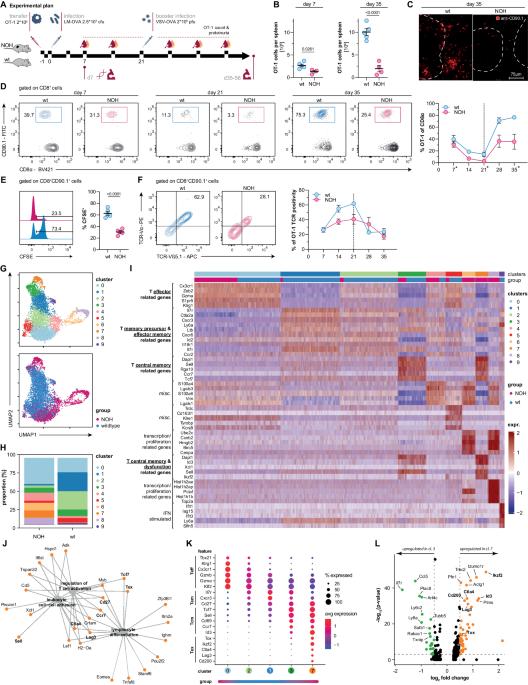
Immune-mediated nephritis is a leading cause of acute kidney injury and chronic kidney disease. While the role of B cells and antibodies has been extensively investigated in the past, the advent of immune-checkpoint inhibitors has led to a reappraisal of the role of T cells in renal immunology. However, it remains elusive how T cells with specificity for renal autoantigens are activated and participate in immune-mediated nephritis. Here, we followed the fate and function of pathogen-activated autoreactive CD8 T cells that are specific for a renal autoantigen. We demonstrate that recently activated splenic CD8 T cells developed a hybrid phenotype in the context of renal autoantigen cross-presentation, combining hallmarks of activation and T cell dysfunction. While circulating memory T cells rapidly disappeared, tissue-resident memory T cells emerged and persisted within the kidney, orchestrating immune-mediated nephritis. Notably, T cells infiltrating kidneys of patients with interstitial nephritis also expressed key markers of tissue residency. This study unveils how a tissue-specific immune response can dissociate from its systemic counterpart driving a compartmentalized immune response in the kidneys of mice and man. Consequently, targeting tissue-resident memory T cells emerges as a promising strategy to control immune-mediated kidney disease.
组织驻留记忆 T 细胞打破对肾脏自身抗原的耐受,并协调免疫介导的肾炎。
免疫介导的肾炎是急性肾损伤和慢性肾病的主要病因。虽然过去对 B 细胞和抗体的作用进行了广泛研究,但免疫检查点抑制剂的出现促使人们重新评估 T 细胞在肾脏免疫学中的作用。然而,对肾脏自身抗原具有特异性的 T 细胞是如何被激活并参与免疫介导的肾炎的仍是个谜。在这里,我们跟踪了病原体激活的对肾脏自身抗原特异的自身反应性 CD8 T 细胞的命运和功能。我们证明,最近激活的脾脏 CD8 T 细胞在肾脏自身抗原交叉呈递的背景下形成了一种混合表型,结合了激活和 T 细胞功能障碍的特征。在循环记忆 T 细胞迅速消失的同时,组织驻留记忆 T 细胞出现并持续存在于肾脏中,协调免疫介导的肾炎。值得注意的是,浸润间质性肾炎患者肾脏的 T 细胞也表达了组织驻留的关键标记。这项研究揭示了组织特异性免疫反应如何与全身性免疫反应相分离,从而在小鼠和人类肾脏中产生分区免疫反应。因此,靶向组织驻留记忆 T 细胞是控制免疫介导的肾脏疾病的一种有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


