YAP represses the TEAD–NF-κB complex and inhibits the growth of clear cell renal cell carcinoma
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The Hippo pathway is generally understood to inhibit tumor growth by phosphorylating the transcriptional cofactor YAP to sequester it to the cytoplasm and reduce the formation of YAP-TEAD transcriptional complexes. Aberrant activation of YAP occurs in various cancers. However, we found a tumor-suppressive function of YAP in clear cell renal cell carcinoma (ccRCC). Using cell cultures, xenografts, and patient-derived explant models, we found that the inhibition of upstream Hippo-pathway kinases MST1 and MST2 or expression of a constitutively active YAP mutant impeded ccRCC proliferation and decreased gene expression mediated by the transcription factor NF-κB. Mechanistically, the NF-κB subunit p65 bound to the transcriptional cofactor TEAD to facilitate NF-κB–target gene expression that promoted cell proliferation. However, by competing for TEAD, YAP disrupted its interaction with NF-κB and prompted the dissociation of p65 from target gene promoters, thereby inhibiting NF-κB transcriptional programs. This cross-talk between the Hippo and NF-κB pathways in ccRCC suggests that targeting the Hippo-YAP axis in an atypical manner—that is, by activating YAP—may be a strategy for slowing tumor growth in patients.
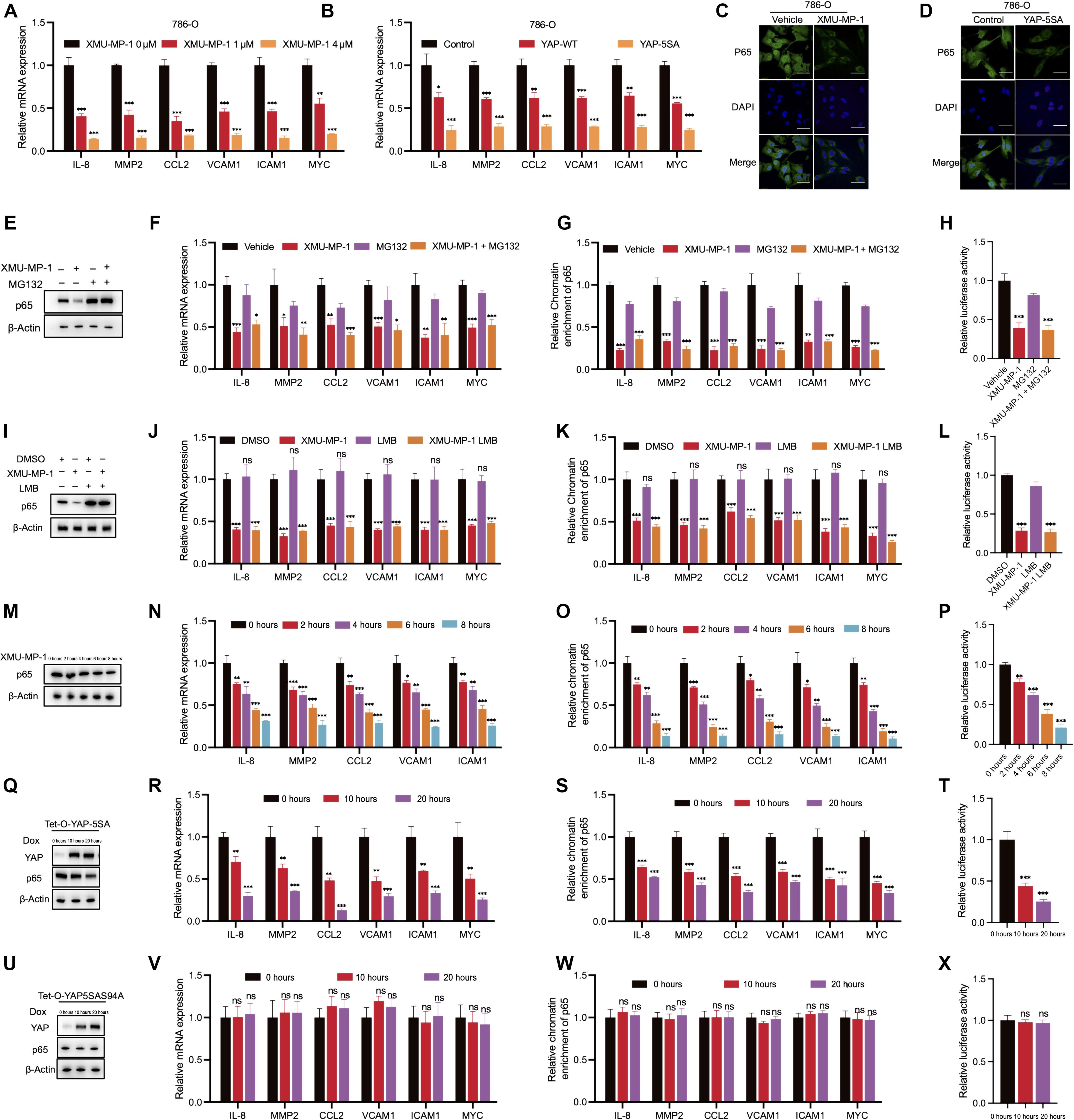
YAP 可抑制 TEAD-NF-κB 复合物并抑制透明细胞肾细胞癌的生长。
一般认为,Hippo 通路通过磷酸化转录辅助因子 YAP,将其封闭在细胞质中,减少 YAP-TEAD 转录复合物的形成,从而抑制肿瘤生长。YAP的异常活化发生在各种癌症中。然而,我们发现 YAP 在透明细胞肾细胞癌(ccRCC)中具有抑制肿瘤的功能。通过使用细胞培养物、异种移植和源自患者的外植体模型,我们发现抑制上游Hippo通路激酶MST1和MST2或表达组成型活性YAP突变体会阻碍ccRCC的增殖,并降低由转录因子NF-κB介导的基因表达。从机制上讲,NF-κB 亚基 p65 与转录辅助因子 TEAD 结合,促进 NF-κB 靶基因的表达,从而促进细胞增殖。然而,通过竞争 TEAD,YAP 破坏了它与 NF-κB 的相互作用,促使 p65 从靶基因启动子解离,从而抑制了 NF-κB 的转录程序。ccRCC中Hippo和NF-κB通路之间的这种交叉对话表明,以非典型方式(即通过激活YAP)靶向Hippo-YAP轴可能是减缓患者肿瘤生长的一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


