B cell lymphoma 6 promotes hepatocellular carcinoma progression by inhibiting tumor infiltrating CD4+T cell cytotoxicity through ESM1
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
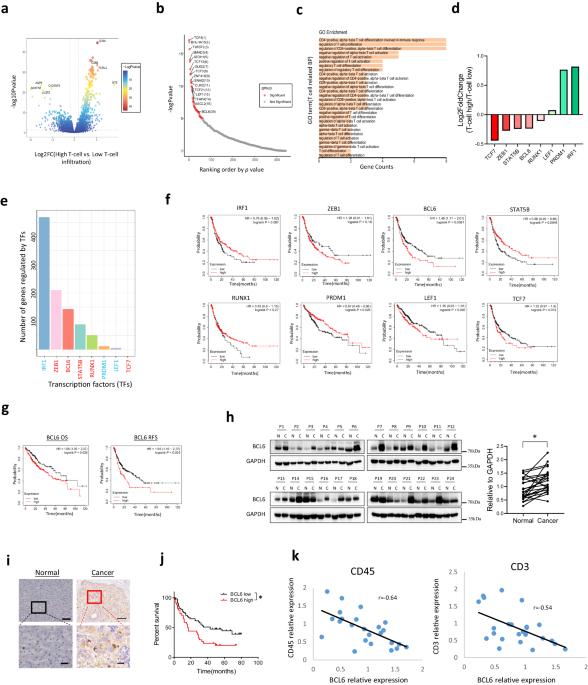
Immunotherapy exhibited potential effects for advanced hepatocellular carcinoma, unfortunately, the clinical benefits are often countered by cancer adaptive immune suppressive response. Uncovering the mechanism how cancer cells evade immune surveillance would help to develop new immunotherapy approaches and combination therapy. In this article, by analyzing the transcriptional factors which modulate the differentially expressed genes between T cell infiltration high group and low group, we identified oncoprotein B cell lymphoma 6 (BCL6) suppresses the infiltration and activation of tumor infiltrating T lymphocytes, thus correlated with poorer clinical outcome. By using antibody deletion experiment, we further demonstrated that CD4+T cells but not CD8+T cells are the main lymphocyte population suppressed by Bcl6 to promote HCC development. Mechanistically, BCL6 decreases cancer cell expression of pro-inflammatory cytokines and T lymphocyte chemokines such as IL6, IL1F6, and CCL5. Moreover, BCL6 upregulates Endothelial cell-specific molecule 1 (ESM1) to inhibit T lymphocyte recruitment and activation possibly through ICAM-1/LFA-1 signaling pathway. Our findings uncovered an unappreciated paracrine mechanism how cancer cell-derived BCL6 assists cancer cell immune evasion, and highlighted the role of CD4+T cells in HCC immune surveillance.
B 细胞淋巴瘤 6 通过 ESM1 抑制肿瘤浸润的 CD4+T 细胞的细胞毒性,从而促进肝细胞癌的进展。
免疫疗法对晚期肝细胞癌具有潜在疗效,但不幸的是,其临床疗效往往被癌症的适应性免疫抑制反应所抵消。揭示癌细胞逃避免疫监视的机制有助于开发新的免疫疗法和联合疗法。本文通过分析调控T细胞浸润高组和低组间差异表达基因的转录因子,发现肿瘤蛋白B细胞淋巴瘤6(BCL6)抑制肿瘤浸润T淋巴细胞的浸润和活化,从而与较差的临床预后相关。通过抗体缺失实验,我们进一步证实了CD4+T细胞而非CD8+T细胞是被BCL6抑制的主要淋巴细胞群,从而促进了HCC的发展。从机理上讲,BCL6 可降低癌细胞促炎细胞因子和 T 淋巴细胞趋化因子(如 IL6、IL1F6 和 CCL5)的表达。此外,BCL6 可能通过 ICAM-1/LFA-1 信号通路上调内皮细胞特异性分子 1(ESM1),从而抑制 T 淋巴细胞的募集和活化。我们的研究结果揭示了癌细胞衍生的BCL6如何协助癌细胞逃避免疫的一种未被重视的旁分泌机制,并强调了CD4+T细胞在HCC免疫监视中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


