Zhao Cheng, Darren P Ennis, Bingxin Lu, Hasan B Mirza, Chishimba Sokota, Baljeet Kaur, Naveena Singh, Olivia Le Saux, Giorgia Russo, Gaia Giannone, Laura A Tookman, Jonathan Krell, Chris Barnes, Jackie McDermott, Iain A McNeish
下载PDF
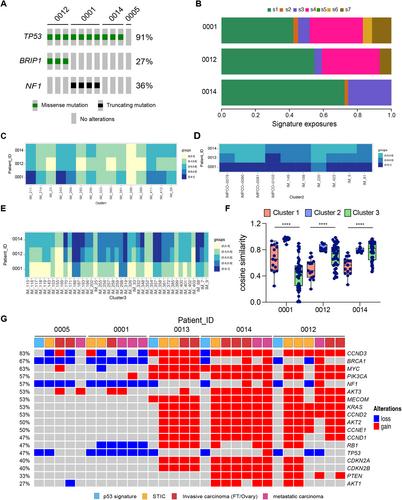
{"title":"The genomic trajectory of ovarian high-grade serous carcinoma can be observed in STIC lesions","authors":"Zhao Cheng, Darren P Ennis, Bingxin Lu, Hasan B Mirza, Chishimba Sokota, Baljeet Kaur, Naveena Singh, Olivia Le Saux, Giorgia Russo, Gaia Giannone, Laura A Tookman, Jonathan Krell, Chris Barnes, Jackie McDermott, Iain A McNeish","doi":"10.1002/path.6322","DOIUrl":null,"url":null,"abstract":"<p>Ovarian high-grade serous carcinoma (HGSC) originates in the fallopian tube, with secretory cells carrying a <i>TP53</i> mutation, known as p53 signatures, identified as potential precursors. p53 signatures evolve into serous tubal intraepithelial carcinoma (STIC) lesions, which in turn progress into invasive HGSC, which readily spreads to the ovary and disseminates around the peritoneal cavity. We recently investigated the genomic landscape of early- and late-stage HGSC and found higher ploidy in late-stage (median 3.1) than early-stage (median 2.0) samples. Here, to explore whether the high ploidy and possible whole-genome duplication (WGD) observed in late-stage disease were determined early in the evolution of HGSC, we analysed archival formalin-fixed paraffin-embedded (FFPE) samples from five HGSC patients. p53 signatures and STIC lesions were laser-capture microdissected and sequenced using shallow whole-genome sequencing (sWGS), while invasive ovarian/fallopian tube and metastatic carcinoma samples underwent macrodissection and were profiled using both sWGS and targeted next-generation sequencing. Results showed highly similar patterns of global copy number change between STIC lesions and invasive carcinoma samples within each patient. Ploidy changes were evident in STIC lesions, but not p53 signatures, and there was a strong correlation between ploidy in STIC lesions and invasive ovarian/fallopian tube and metastatic samples in each patient. The reconstruction of sample phylogeny for each patient from relative copy number indicated that high ploidy, when present, occurred early in the evolution of HGSC, which was further validated by copy number signatures in ovarian and metastatic tumours. These findings suggest that aberrant ploidy, suggestive of WGD, arises early in HGSC and is detected in STIC lesions, implying that the trajectory of HGSC may be determined at the earliest stages of tumour development. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 1","pages":"42-54"},"PeriodicalIF":5.6000,"publicationDate":"2024-07-02","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6322","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6322","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Ovarian high-grade serous carcinoma (HGSC) originates in the fallopian tube, with secretory cells carrying a TP53 mutation, known as p53 signatures, identified as potential precursors. p53 signatures evolve into serous tubal intraepithelial carcinoma (STIC) lesions, which in turn progress into invasive HGSC, which readily spreads to the ovary and disseminates around the peritoneal cavity. We recently investigated the genomic landscape of early- and late-stage HGSC and found higher ploidy in late-stage (median 3.1) than early-stage (median 2.0) samples. Here, to explore whether the high ploidy and possible whole-genome duplication (WGD) observed in late-stage disease were determined early in the evolution of HGSC, we analysed archival formalin-fixed paraffin-embedded (FFPE) samples from five HGSC patients. p53 signatures and STIC lesions were laser-capture microdissected and sequenced using shallow whole-genome sequencing (sWGS), while invasive ovarian/fallopian tube and metastatic carcinoma samples underwent macrodissection and were profiled using both sWGS and targeted next-generation sequencing. Results showed highly similar patterns of global copy number change between STIC lesions and invasive carcinoma samples within each patient. Ploidy changes were evident in STIC lesions, but not p53 signatures, and there was a strong correlation between ploidy in STIC lesions and invasive ovarian/fallopian tube and metastatic samples in each patient. The reconstruction of sample phylogeny for each patient from relative copy number indicated that high ploidy, when present, occurred early in the evolution of HGSC, which was further validated by copy number signatures in ovarian and metastatic tumours. These findings suggest that aberrant ploidy, suggestive of WGD, arises early in HGSC and is detected in STIC lesions, implying that the trajectory of HGSC may be determined at the earliest stages of tumour development. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


