TRIM65 deficiency alleviates renal fibrosis through NUDT21-mediated alternative polyadenylation
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Chronic kidney disease (CKD) is a major global health concern and the third leading cause of premature death. Renal fibrosis is the primary process driving the progression of CKD, but the mechanisms behind it are not fully understood, making treatment options limited. Here, we find that the E3 ligase TRIM65 is a positive regulator of renal fibrosis. Deletion of TRIM65 results in a reduction of pathological lesions and renal fibrosis in mouse models of kidney fibrosis induced by unilateral ureteral obstruction (UUO)- and folic acid. Through screening with a yeast-hybrid system, we identify a new interactor of TRIM65, the mammalian cleavage factor I subunit CFIm25 (NUDT21), which plays a crucial role in fibrosis through alternative polyadenylation (APA). TRIM65 interacts with NUDT21 to induce K48-linked polyubiquitination of lysine 56 and proteasomal degradation, leading to the inhibition of TGF-β1-mediated SMAD and ERK1/2 signaling pathways. The degradation of NUDT21 subsequently altered the length and sequence content of the 3′UTR (3′UTR-APA) of several pro-fibrotic genes including Col1a1, Fn-1, Tgfbr1, Wnt5a, and Fzd2. Furthermore, reducing NUDT21 expression via hydrodynamic renal pelvis injection of adeno-associated virus 9 (AAV9) exacerbated UUO-induced renal fibrosis in the normal mouse kidneys and blocked the protective effect of TRIM65 deletion. These findings suggest that TRIM65 promotes renal fibrosis by regulating NUDT21-mediated APA and highlight TRIM65 as a potential target for reducing renal fibrosis in CKD patients.
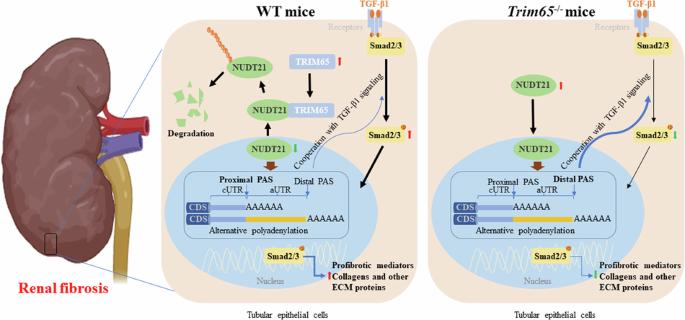
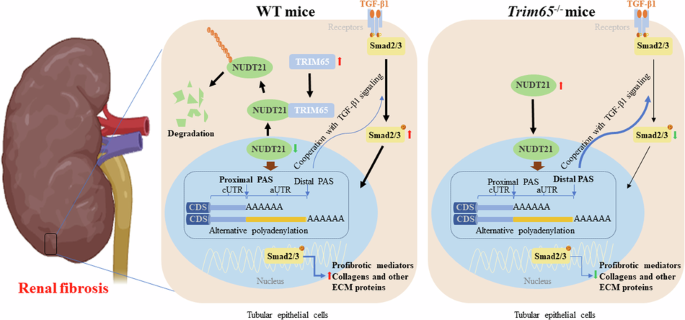
TRIM65的缺乏可通过NUDT21介导的替代多腺苷酸化减轻肾脏纤维化。
慢性肾脏病(CKD)是全球关注的主要健康问题,也是导致过早死亡的第三大原因。肾脏纤维化是推动 CKD 进展的主要过程,但其背后的机制尚未完全明了,因此治疗方案有限。在这里,我们发现 E3 连接酶 TRIM65 是肾脏纤维化的正向调节因子。在单侧输尿管梗阻(UUO)和叶酸诱导的肾脏纤维化小鼠模型中,TRIM65的缺失会导致病理病变和肾脏纤维化的减少。通过酵母杂交系统的筛选,我们发现了TRIM65的一个新的互作因子--哺乳动物裂解因子I亚基CFIm25(NUDT21),它通过替代多腺苷酸化(APA)在肾脏纤维化中发挥关键作用。TRIM65 与 NUDT21 相互作用,诱导赖氨酸 56 的 K48 链接多泛素化和蛋白酶体降解,从而抑制 TGF-β1 介导的 SMAD 和 ERK1/2 信号通路。NUDT21 的降解随后改变了几个促纤维化基因(包括 Col1a1、Fn-1、Tgfbr1、Wnt5a 和 Fzd2)3'UTR(3'UTR-APA)的长度和序列内容。此外,通过肾盂水动力注射腺相关病毒9(AAV9)减少NUDT21的表达会加剧UUO诱导的正常小鼠肾脏纤维化,并阻断TRIM65缺失的保护作用。这些研究结果表明,TRIM65通过调节NUDT21介导的APA促进肾脏纤维化,并强调TRIM65是减轻慢性肾脏病患者肾脏纤维化的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


