USP18 promotes colon adenocarcinoma progression via targeting the ERK-MNK signaling pathway
Abstract
Background
Colorectal cancer is the third most common malignancy worldwide and is one of the leading causes of cancer-related mortality. Ubiquitin-specific peptidase 18 (USP18) protein has been reported to exert different tumor-related effects in distinct tumor types. Here, we initially investigated the expression and signaling pathways of USP18 in colon adenocarcinoma (COAD).
Methods
A quantitative real-time PCR was conducted to evaluate the mRNA level of USP18 in cultured cells. Immunohistochemical staining was used to explore the protein expression of USP18 in clinical COAD samples. Specific knockdown was achieved by transient transfection of small interfering RNAs into SW480 and HT29 cells using Lipo3000. Cell conting kit-8 assay, transwell assay and matrigel-transwell assays were conducted to evaluate proliferation, migration and invasion capacities, respectively. Western blotting was performed to analyze downstream signaling pathways. A chi-squared test and univariate and multivariate analyses were used to evaluate the clinical data. Xenografts from mice model were assessed to validate the in vitro findings.
Results
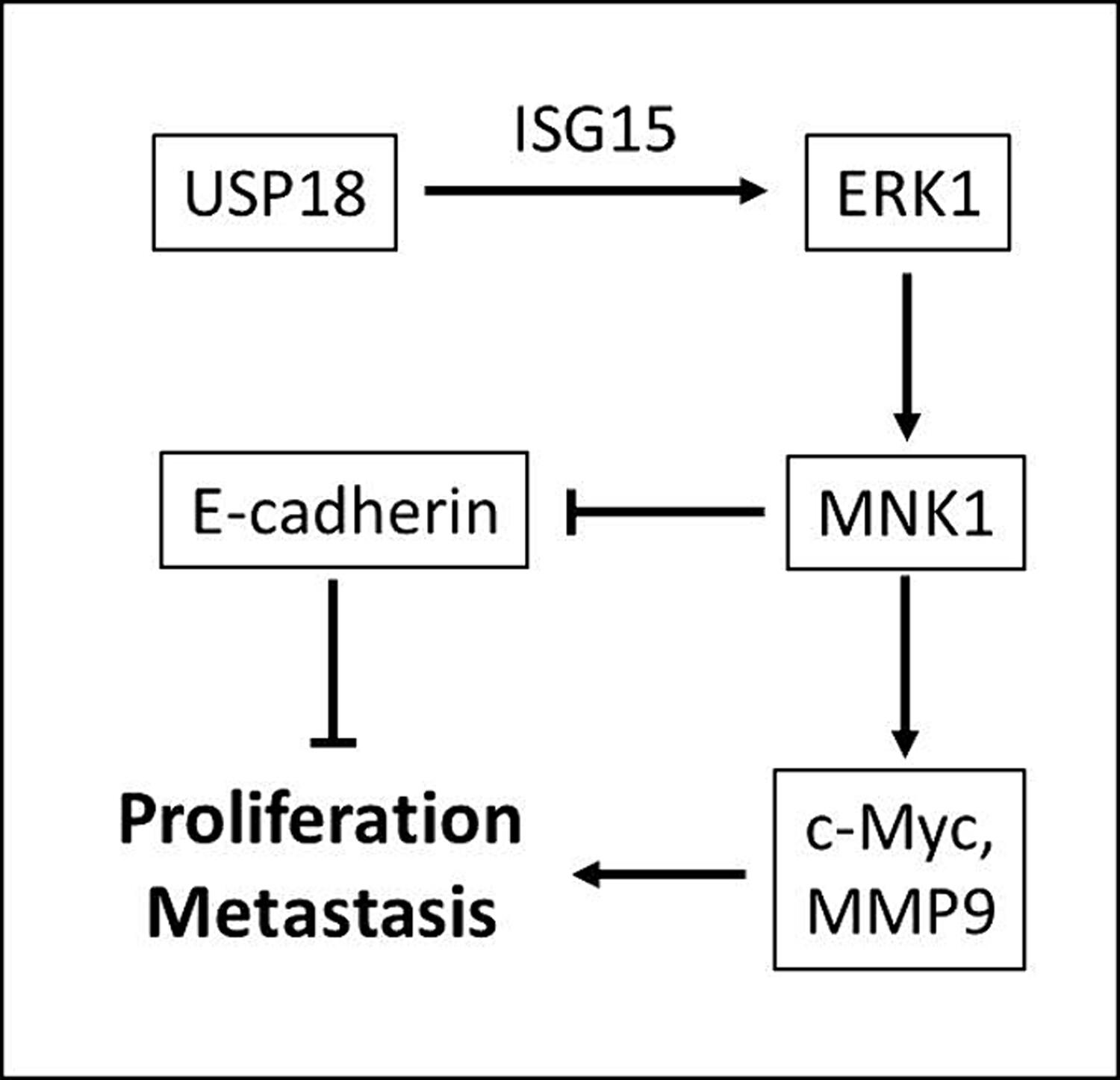
Higher USP18 level was identified in COAD tissues and was positively correlated with advanced tumor stage. High USP18 protein expression indicated poorer prognosis of COAD patients. Silencing USP18 suppressed COAD cell proliferation and invasion via destabilizing extracellular signal-regulated kinase (ERK) protein and suppressing ERK downstream pathways. Simultaneously silencing interferon-stimulated gene 15 (ISG15) with USP18 can partially rescue the tumor cell viability, indicating its involvement in USP18 signaling. The oncogenic effects of USP18 were also confirmed in mice models.
Conclusions
USP18 plays oncogenic effects in colon adenocarcinoma via ISG15-ERK pathways. High USP18 expression indicates poor clinical outcomes for colon adenocarcinoma patients.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


