Enhancing cognitive recovery in chronic traumatic brain injury through simultaneous allosteric modulation of α7 nicotinic acetylcholine and α5 GABAA receptors
Abstract
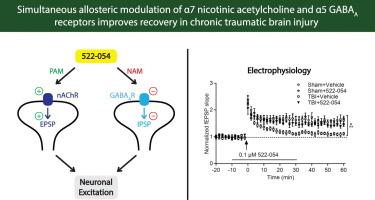
Traumatic brain injury (TBI) leads to changes in the neural circuitry of the hippocampus that result in chronic learning and memory deficits. However, effective therapeutic strategies to ameliorate these chronic learning and memory impairments after TBI are limited. Two pharmacological targets for enhancing cognition are nicotinic acetylcholine receptors (nAChRs) and GABAA receptors (GABAARs), both of which regulate hippocampal network activity to form declarative memories. A promising compound, 522–054, both allosterically enhances α7 nAChRs and inhibits α5 subunit-containing GABAARs. Administration of 522–054 enhances long-term potentiation (LTP) and cognitive functioning in non-injured animals. In this study, we assessed the effects of 522–054 on hippocampal synaptic plasticity and learning and memory deficits in the chronic post-TBI recovery period. Adult male Sprague Dawley rats received moderate parasagittal fluid-percussion brain injury or sham surgery. At 12 wk after injury, we assessed basal synaptic transmission and LTP at the Schaffer collateral-CA1 synapse of the hippocampus. Bath application of 522–054 to hippocampal slices reduced deficits in basal synaptic transmission and recovered TBI-induced impairments in LTP. Moreover, treatment of animals with 522–054 at 12 wk post-TBI improved cue and contextual fear memory and water maze acquisition and retention without a measurable effect on cortical or hippocampal atrophy. These results suggest that dual allosteric modulation of α7 nAChR and α5 GABAAR signaling may be a potential therapy for treating cognitive deficits during chronic recovery from TBI.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


