BAG6 restricts pancreatic cancer progression by suppressing the release of IL33-presenting extracellular vesicles and the activation of mast cells
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Recent studies reveal a critical role of tumor cell-released extracellular vesicles (EVs) in pancreatic cancer (PC) progression. However, driver genes that direct EV function, the EV-recipient cells, and their cellular response to EV uptake remain to be identified. Therefore, we studied the role of Bcl-2-associated-anthanogene 6 (BAG6), a regulator of EV biogenesis for cancer progression. We used a Cre recombinase/LoxP-based reporter system in combination with single-cell RNA sequencing to monitor in vivo EV uptake and tumor microenvironment (TME) changes in mouse models for pancreatic ductal adenocarcinoma (PDAC) in a Bag6 pro- or deficient background. In vivo data were validated using mouse and human organoids and patient samples. Our data demonstrated that Bag6-deficient subcutaneous and orthotopic PDAC tumors accelerated tumor growth dependent on EV release. Mechanistically, this was attributed to mast cell (MC) activation via EV-associated IL33. Activated MCs promoted tumor cell proliferation and altered the composition of the TME affecting fibroblast polarization and immune cell infiltration. Tumor cell proliferation and fibroblast polarization were mediated via the MC secretome containing high levels of PDGF and CD73. Patients with high BAG6 gene expression and high protein plasma level have a longer overall survival indicating clinical relevance. The current study revealed a so far unknown tumor-suppressing activity of BAG6 in PDAC. Bag6-deficiency allowed the release of EV-associated IL33 which modulate the TME via MC activation promoting aggressive tumor growth. MC depletion using imatinib diminished tumor growth providing a scientific rationale to consider imatinib for patients stratified with low BAG6 expression and high MC infiltration.
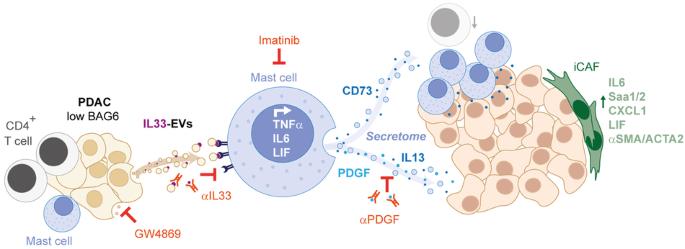
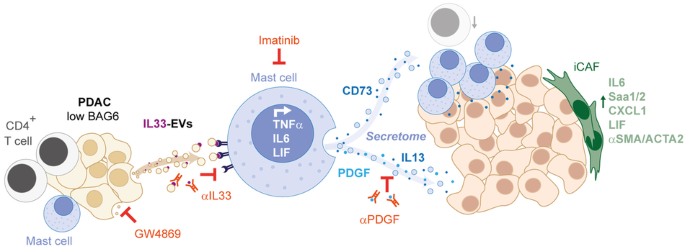
BAG6 通过抑制 IL33 呈递细胞外囊泡的释放和肥大细胞的活化来限制胰腺癌的进展。
最近的研究表明,肿瘤细胞释放的细胞外囊泡(EV)在胰腺癌(PC)进展中起着关键作用。然而,指导 EV 功能的驱动基因、EV 接收细胞及其对 EV 吸收的细胞反应仍有待确定。因此,我们研究了 Bcl-2-associated-anthanogene 6(BAG6)在癌症进展中的作用,它是 EV 生物发生的调控因子。我们使用基于 Cre 重组酶/LoxP 的报告系统,结合单细胞 RNA 测序,在 Bag6 亲合或缺乏背景的胰腺导管腺癌(PDAC)小鼠模型中监测体内 EV 摄取和肿瘤微环境(TME)变化。使用小鼠和人类器官组织以及患者样本对体内数据进行了验证。我们的数据表明,Bag6缺陷的皮下和正位PDAC肿瘤依赖于EV的释放而加速肿瘤生长。从机理上讲,这是由于肥大细胞(MC)通过EV相关的IL33被激活。活化的肥大细胞促进了肿瘤细胞的增殖,并改变了TME的组成,影响了成纤维细胞的极化和免疫细胞的浸润。肿瘤细胞增殖和成纤维细胞极化是通过含有高水平PDGF和CD73的MC分泌组介导的。高BAG6基因表达和高蛋白血浆水平的患者总生存期更长,这表明该研究具有临床意义。目前的研究揭示了 BAG6 在 PDAC 中迄今未知的肿瘤抑制活性。Bag6缺失会导致EV相关的IL33释放,而IL33会通过激活MC调节TME,促进肿瘤的侵袭性生长。使用伊马替尼清除MC可减少肿瘤生长,这为考虑对BAG6低表达和MC高浸润的患者使用伊马替尼提供了科学依据。从BAG6缺陷的胰腺癌细胞中提取的EV通过IL33/Il1rl1诱导MC活化。活化的MC的分泌物诱导肿瘤增殖和TME变化,尤其是使成纤维细胞转变为炎症性癌症相关成纤维细胞(iCAF)表型。阻断EVs或消耗MCs可限制肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


